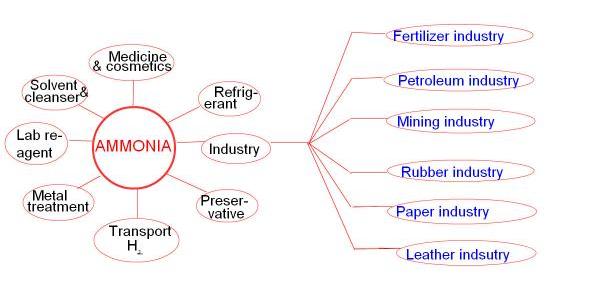
Ammonia
source : en.wikipedia.org
Chemical properties:
1. Combustion:
when mixture of NH3 and O2 burns, it gives N2 gas.
4NH3 + 3O2 ——–> 2 N2 + 6H2O
2. Basic nature:
a) Aqueous solution of NH3 is NH4OH which is base & turns red litmus blue.
NH3 +H2O ——> NH4OH
b) Ammonia reacts with acid to form salt:
NH3 +HCl ——–> NH4Cl
2NH3 + H2SO4 ——-> (NH4)2SO4
NH3 + HNO3 —–> NH4NO3 (Ammonium nitrate)
c) There is one lone pair of electron present on nitrogen atom of NH3. Therefore NH3 acts as electron pair donor and is a Lewis base.
:NH3 (Lewis base)
d) Reaction with halogens :
i) Reaction with chlorine :
When chlorine reacts with excess of ammonia then nitrogen & ammonium chloride is formed.
2NH3 +3 Cl2 ——> N2 + 6HCl
6HCl + 6 NH3 ——> 6NH4Cl
Reaction as a whole,
8NH3 + 3Cl2 ——> N2 + 6NH4Cl
when excess of chlorine reacts with ammonia then Nitrogen tri chloride is formed.
NH3 + 3Cl2 ——–> NCl3 (explosive) + 3HCl
ii) Reaction with Bromine:
2NH3 + 3Br2 ———> N2 + 6HBr
6HBr + 6NH3 ——-> 6NH4Br
Reaction as a whole is ,
8NH3 + 3Br2 —–> N2 + 6NH4Br
iii) Reaction with Iodine:
NH3 + I2 ———-> NH3.NI3 +NH4I
NH3.NI3 ——-> NH4I +N2 +I2
e) Reaction with CuO or PbO:
2NH3 +3 CuO ———> 3Cu + N2 +3H2O
3PbO +2NH3 ——–> 3Pb +N2 +3H2O
f) Reaction with FeCl3:
FeCl3 + 3NH4OH ——>Fe(OH)3 (red-brown precipitate of ferric hydroxide) +3NH4Cl
g) Reaction with CrCl3:
CrCl3+ 3NH4OH ——–> Cr(OH)3 [chromium hydroxide(insoluble)]+ 3NH4Cl
we know that Cr(OH)3 has green precipitate.
h) Reaction with AlCl3:
AlCl3 + 3NH4OH ——-> Al(OH)3 (white precipitate of Aluminium hydroxide) +3NH4Cl
i) Reaction with AgCl:
with small amount of NH4OH precipitate comes out which is soluble in excess of NH4OH due to complex formation.
2AgCl + 2NH4OH ——-> Ag2O +H2O + 2NH4Cl
Ag2O + NH4OH +NH4Cl ———> [Ag(NH3)2]Cl [diamine silver (I) chloride (soluble complex)]+H2O
j) Reaction with AgNO3:
AgNO3 + 2NH4OH ——->[ Ag(NH3)2 ]NO3 [diamine silver(I) nitrate] + 2H2O
k) Reaction with Copper sulphate:
with the small amount of NH4OH, blue precipitate of cupric hydroxide is obtained. Which is soluble in excess of NH4OH due to formation of soluble complex.
CuSO4 + 2NH4OH ——–> Cu(OH)2 [blue precipitate ]+(NH4)2SO4
Cu(OH)2 + 2NH4OH + (NH4)2SO4 ——>[ Cu(NH3)4 ]SO4 + 4H2O
[Cu(NH3)4]SO4 is tetra amine copper (II) sulphate (Soluble blue complex).
l) Reaction with ZnSO4:
ZnSO4 + 2NH4OH ——–> Zn(OH)2 (white precipitate) + (NH4)2SO4
Zn(OH)2 + 2NH4OH + (NH4)2SO4 ——->[ Zn(NH3)4]SO4 + 4H2O
[Zn(NH3)4]SO4 is tetra amine zinc (II) sulphate (soluble colorless complex).
m) Reaction with ZnCl2:
ZnCl2 + 2NH4OH ——–> Zn(OH)2 +2NH4Cl
Zn(OH)2 + 2NH4OH +2NH4Cl ——->[ Zn(NH3)4 ]Cl2 + 4 H2O
[Zn(NH3)4 ]Cl2 is tetra amine zinc (II) chloride (soluble colorless complex).
n) Reaction with CdSO4:
CdSO4 + 2NH4OH ——-> Cd(OH)2 +(NH4)2SO4
Cd(OH)2 + 2NH4OH +(NH4)2SO4 —–> [ Cd(NH3)4]SO4 + 4H2O
[Cd(NH3)4 ]SO4 is tetra amine cadmium (II) sulphate (soluble colorless complex).
o) Reaction with Mercurous chloride:
Black residue of mercuric amino chloride and mercury is obtained.
Hg2Cl2 +2 NH4OH ——-> Cl-Hg-NH2 (mercuric amino chloride)+ Hg + 2H2O +NH4Cl
p) Reaction with mercuric chloride:
HgCl2 + 2NH4OH ——–> Cl-Hg-NH2 (white color) +NH4Cl + 2H2O