Carbonium ion

source : difference between.com
Carbonium ion or carbocation-
When a covalent bond, in which Carbon is linked to more electronegative atom or group, breaks up by heterolytic fission . The more electronegative atom takes away the electron pair while carbon loses its electron & thus carbon acquires a positive charge . These are obtained by heterolytic fission.

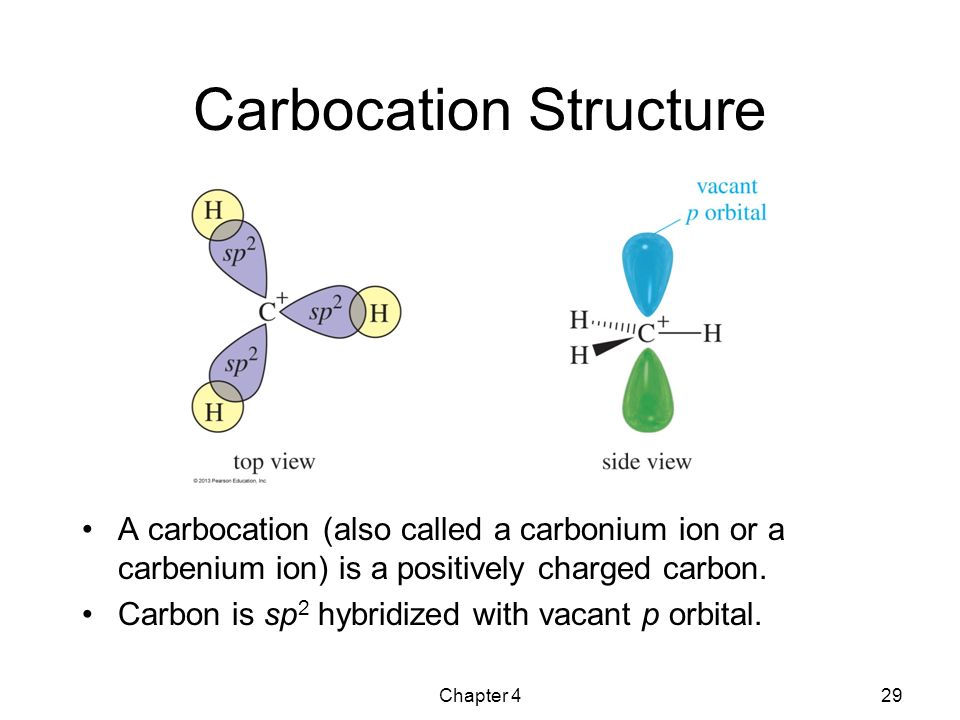
“Such organic ions carrying a positive charge on Carbon-atom are known as Carbonium ion or Carbocations.”
These are named by adding the ‘Carbonium ion’ after the name of parent alkyl group . There are also termed as Primary , secondary & tertiary depending upon the nature of Carbon-atom bearing positive charge .

Characteristics :
1) Carbon-atom of the carbonium ion contains positive charge.
2) Carbon-atom carrying positive charge has six electrons in its valence shell i.e., two electrons less than octet.
3) The positively charged carbon-atom of carbonium ion is in sp2 hybridization state. Its shape is planar. It uses sp2 hybrid orbitals to form three sigma bonds .
4) These are very reactive. They readily react with the groups which can donate a pair of electron . (example – nucleophiles)

5) Carbocation is itself an electrophile
6) The order of stability of carbonium ions are as follows-

7) The presence of a group having -I effect such as (-Cl) decreases the stability & increases the reactivity.
On the other hand, the presence of a group having +I effect , in a carbocation increases stability and decreases its reactivity.

8)The stability of a carbocation is influenced by resonance. Both allyl & benzyl carbonium ions possess resonance & thus extra stable.