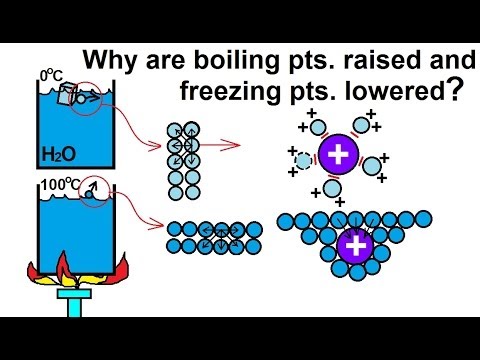
Depression in freezing point

source : surfguppy.com
Depression in freezing point –
Question 1) It has been found that minimum temperature recorded in a hill station is -10 0 C. Calculate the amount of glycerine to be added to 40 dm3 water used in car radiator, so that it does not freeze. Δ H fusion = 6.01 KJ/mole
Solution )
K = 1.86
m of glycerine (CH2 OH-CHOH-CH2OH) =92
ΔTf =freezing point of solvent – freezing point of solution
freezing point of solvent = 0 0C
freezing point of solution = – 10 0C
Depression in freezing point ΔTf = 0 – (-10) = 10
Volume of solvent ‘V’ = 40 dm3 =40 x 1000 =40000 cc
weight of solvent ‘W’ = volume x density
weight of solvent ‘W’= 40000 x 1=40000 gm
w = ΔTf .W.m/1000 Kf
w = 10 x 40000 x 92 /1000 x 1.86
w= 19784.94 gm
w = 19.78 Kg. Ans.
Question 2) Calculate the amount of ice that will separate out on cooling a solution containing 50 gm of ethylene glycol in 200 gm of water to -9.3 0C. (Kf for water =1.86 K Kg /mole )
Solution )
Kf = 1.86
m of ethylene glycol (CH2OH-CH2OH) = 62
ΔTf =freezing point of solvent – freezing point of solution
freezing point of solvent = 0 0C
freezing point of solution = – 9.3 0C
ΔTf = 0 – (-9.3) = 9.3
w =50 gm.
W =1000 Kf. w /ΔTf .m
W= 1000 x 1.86 x 50 / 9.3 x 62
W( weight of solvent ) = 161.29 gm.
Total solvent taken = 200 gm.
weight of ice separated =200 – 161.29 = 38.71 gm. Ans.
Question 3) If boiling point of an aqueous solution is 100.1 0 C. What is its freezing point ? Given latent heat of fusion and vaporisation of water are 80 calorie/gm and 540 calorie /gm respectively.
Solution )
ΔTf =Kf x molality
ΔTb =Kb x molality
ΔTb / ΔTf =Kb x molality / Kf x molality
ΔTb / ΔTf = Kb /Kf
ΔTb / ΔTf =( R Tb2 /1000 x lv ) / ( R Tf2 /1000 x lf )
ΔTb / ΔTf = Tb2 x lf / lv x Tf2
lv = 540 calorie /gm
lf = 80 calorie /gm
Boiling point of solution =100.1 0C =100.1 + 273 =373.1 K
Boiling point of solvent =100 0 C
Tf = freezing point of solvent (water)=0 0 C =0 + 273 = 273 K
Tb =Boiling point of solvent (water) = 100 0C = 100 + 273 = 373 K
ΔTb / ΔTf = Tb2 x lf / lv x Tf2
(373.1 – 373 ) / ΔTf = 373 x 373 x 80 /540 x 273 x 273
depression in freezing point (ΔTf )= 0.36
ΔTf =freezing point of solvent – freezing point of solution
freezing point of solution = freezing point of solvent – ΔTf
freezing point of solution= 273 – 0.36 =272.64 K or – 0.36 0C Ans.
Question 4) 0.48 gm of an organic compound dissolved in 10.6 gm of benzene lowered the freezing point by 1.8 0 C . find the molecular weight of compound. ( molecular depression constant for benzene = 50 )
Solution )
w = 0.48 gm , ΔTf =1.8 0C , Kf = 50 ,W =10.6 gm , m = ?
m =100 Kf. w /ΔTf .W
m = 100 x 50 x 0.48 / 1.8 x 10.6
m =125.79 Ans.