Hybridization

source : wn.com
Hybridization-
Mixing of atomic orbitals of nearly equal energy and to form new orbitals of equal energy is called hybridization. The new orbitals are known as hybrid orbitals.
Characteristic of hybridization :
- Hybridization of atomic orbitals take place only.
- Atomic orbitals which take part in hybridization may be fulfilled, half filled or vacant.
- Hybrid orbitals always form sigma bond.
- Hybrid orbitals have definite shape and geometry.
Types of hybridization :
sp hybridization :
In this hybridization one s & one p orbital are mixed together and two hybrid orbitals of equal energy are formed. These are called sp hybrid orbital and this process is called sp hybridization.
Properties of sp Hybrid orbital :
- Two sp-hybrid orbitals are equivalent in energy.
- Geometry is linear and angle is 1800.
- sp-hybrid orbital is stronger than pure s and p orbitals.
- sp -hybrid orbital has two lobes.
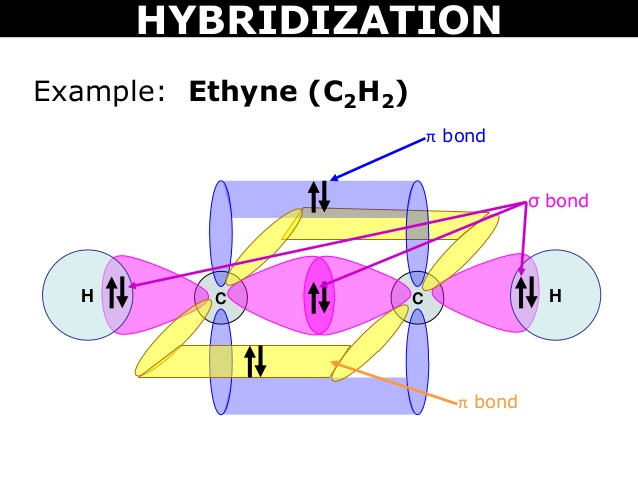
Example: C2H2 , CO2 , BeCl2 , N2O etc.
6C : 1s2,2s2,2p2

source :Assignment help

Geometry is linear and angle is 1800.
source : Adi chemistry.com
Formation of BeCl2 molecule :
Central atom in BeCl2 is Be.
4Be : 1s2, 2s2
17Cl : 1s2,2s2,2p6,3s2,3p5

source : SlideShare
One s and one p orbital of Be hybridization to form two sp hybrid orbitals. Each hybrid orbital overlaps with singly filled p-orbital of chlorine. So two sigma bonds are formed.Geometry of molecule is linear and bond angle is 1800.
sp2 hybridization :
In this hybridization one s, two p orbitals are mixed together and three hybrid orbitals of equal energy are formed. These are called sp2 hybrid orbital and this process is called sp2 hybridization.
Properties of sp2 hybrid orbitals :
- All three hybrid orbitals are equivalent in shape and energy.
- Three hybrid orbitals lie in the same plane and are directed towards three corners of an equilateral triangle.
- Bond angle is 1200.
Example: BF3
In BF3 molecule central atom is B.
5B = 1s2,2s2,2p1

source : Meritnation
One s and two p-orbitals of Boron atom hybridize to form three sp2 hybrid orbitals. These hybrid orbitals are singly occupied and directed towards three corners of an equilateral triangle.
Each hybrid orbital overlaps with singly filled 2pz orbital of F- atom. So three sigma bonds are formed. Geometry of molecule is trigonal or triangular planar and bond angle is 1200.

source : Slideplayer.com