sulphuric acid
Contact process:

source : sds enthil.com
Manufacture of sulphuric acid by Contact Process :
principle :
SO2 is oxidized into SO3 by air. The reaction is reversible and exothermic.
2SO2 + O2 <——> 2SO3 + 45.2 K.Calorie
According to Le-chatelier’s principle, high pressure and low temperature will favor the formation of SO3. The activation of energy of reaction is high. Therefore reaction is slow at low temperature. Pt catalyst is added to lower the activation energy of the reaction. In presence of catalyst optimum temperature of the reaction is 400-450 0C. At high pressure, plant became corroded. Therefore the oxidation of SO2 is carried out at 1.5 to 1.7 atm pressure.
Now-a-days V2O5 is used as catalyst in place of Pt because Pt is a costly metal and poisoned by compound of arsenic. V2O5 is cheap and not poisoned by compounds of arsenic. SO3 is absorbed in concentrated H2SO4 to get fuming sulphuric acid. Sulphuric acid of required concentration is obtained by diluting oleum with water.
SO3 + H2SO4 ——> H2S2O7 (oleum or fuming sulphuric acid)
H2S2O7 + H2O ———–> 2H2SO4
Process of manufacture of sulphuric acid:
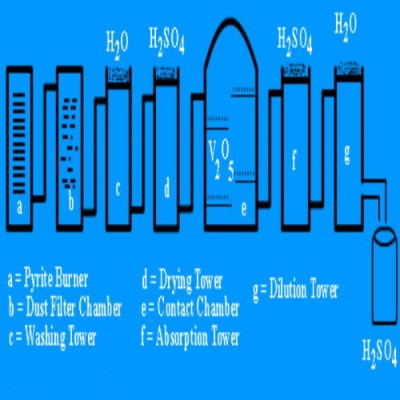
1. Pyrite or sulphur burner:
It is a furnace in which sulphur or iron pyrite are burnt in presence of air to get SO2.
S+ O2 ——-> SO2
4FeS2 + 11O2 —–> 2Fe2O3+ 8SO2
2. Dust chamber :
Mixture of impure SO2 and air obtained from pyrite burner is passed in dust chamber. Stream is passed from the top then dust particles present in the gaseous mixture settle down.
3. Cooler or cooling pipes:
Mixture of SO2 and air is passed through cooling pipes and cooled to 1000 C. The gaseous mixture is passed to washing tower.
4. Washing tower:
It is a lead tower packed with stone pieces. In this tower H2O is spread from the top. The dust particle present are settled down and water soluble impurities get dissolved and removed. The gaseous mixture is send to drying tower.
5. Drying tower:
In this tower, concentrated H2SO4 is spread from the top and moisture present in the wet gaseous mixture is absorbed by concentrated H2SO4. The gaseous mixture is now taken into arsenic purifier.
6. Arsenic purifier:
It is a tower filled with ferric hydroxide. It absorbs compound of arsenic such as As2O3 present as impurity. Dry and arsenic free gaseous mixture is taken into test box.
7. Test box:
It is a empty box in which light is passed. Pure gaseous mixture is transparent and no dust particles are visible then gaseous mixture is pure and taken into converter.
8. Converter or contact chamber:
It is an iron tower packed with Pt or V2O5 catalyst. The temperature of catalyst is 400 – 450 0 in presence of catalyst. SO2 is oxidised by air into SO3. The heat liberated during the reaction maintains the temperature of the catalyst.
2SO2 + O2 <——-> 2SO3 + 45.2 K.Calorie
9. Absorption tower:
SO3 obtained from converter is taken in absorption tower. In this tower, concentration H2SO4 is spread from the top. Tower is packed with stones or quartz pieces. Concentrated H2SO4 absorbs SO3 and forms oleum which is diluted with H2O to get H2SO4 of required concentration.
SO3 + H2SO4 —–> H2S2O7 (oleum or fuming sulphuric acid)
H2S2O7 + H2O —–> 2H2SO4