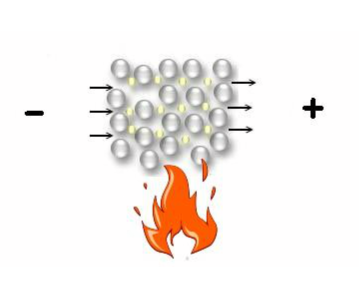
Metallic bond
source : Toondoo.com
Metallic bond-
In the periodic table more than 80 elements are metals. Except Mercury and Gallium all other metals are solid at ordinary temperature. Metals possess characteristic and some unique properties.Such as
- Metals are good conductor of heat and electricity.
- Metals possess a unique lustre called metallic lustre .
- Metals are malleable (can be beaten into sheets) , ductile (can be converted into wire) and possess high tensile strength.
- Some metals are soft like alkali metals (Na, K) while some are hard like tungusten.
Definition-
” The force which binds together the atoms of metals is called Metallic bond.”
Reason –
Ionic bond is not present in metals-
Ionic bond is formed by the transfer of electrons between two atoms . In ionic bond one atom must be electropositive and other must be electronegative.Ionic bond is not formed between similar atoms.Metals conduct electricity in solid state while ionic compounds do not conduct electricity in solid state. Metals are malleable and ductile while ionic compounds are not.
Covalent bond is not present in metals-
Covalent bond is formed by mutual sharing of electrons between two atoms so that the atoms acquire noble gas configuration . In case of metal less electrons are present in valence shell so they are not able to complete their octet by mutual sharing of electrons. Metals are solid while covalent compounds are generally liquid and gases.Metals are good conductor of heat and electricity while covalent compounds are not.
Metals are neither held by ionic bond nor by covalent bond. They have metallic bond .