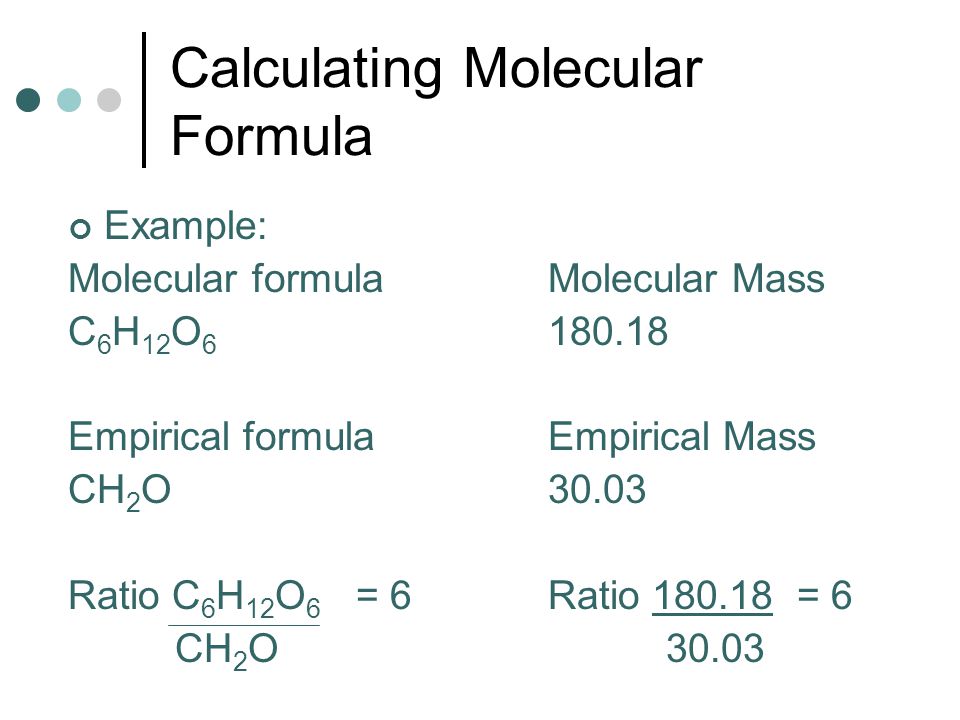
Molecular Formula-

source : slide player.com
Molecular Formula
Q 1–(a) % of C= 40% ; % of H= 6.7%
(b) 0.9 gm. silver salt of monobasic acid on combustion give 0.582 gm.of silver. calculate Molecular Formula = ?
Ans. W= 0.9 gm. , w = 0.582 gm.
% of C= 40% ; % of H= 6.7%
% of O = 100–(40+6.7)=53.3
| Element | % | Atomic weight | Relative no. of atoms | Simplest Ratio |
| C | 40 | 12 | 40/12= 3.3 | 3,3/3.3= 1 |
| H | 6.7 | 1 | 6.7 /1 = 6.7 | 6.7/ 3.3=2 |
| O | 53.3 | 16 | 53.3 /16= 3.3 | 3.3/3.3=1 |
C:H:O=1 : 2 : 1
Empirical formula =CH2O
Empirical formula weight =12+2+16 =30
Calculation of molecular weight by Silver salt method-
E+107/108 =W/w
=0.9/ 0.582
=1.55
E = 167.4 -107 =60.4
molecular weight =E x basicity of acid
=60.4 x 1 = 60.4
n= molecular weight/ Empirical formula weight
n= 60.4 /30 = 2
mol. formula =( Empirical formula )n
=(CH2O)n
=(CH2O)2
mol. formula = C2H4O2 Ans.
Q 2– (a)% of C= 77.42 %, % of H = 7.53,
(b) 0.465 gm. of organic compd is Kjeldhalised then evolved NH3 is completely neutralised by 25 ml N/5 H2SO4. 0.596 gm platini Chloride of a monoacidic base on combustion gave 0.195 gm Pt. calculate the mol. formula of base ?
Ans. % of nitrogen = 1.4 N V/w
N=1/5 =0.2 ; V=25 ml. ,w =0.465 gm.
% of nitrogen =1.4 x 0.2 x 25 /0.465
= 15.05 %
% of O = 100–(77.42 +7.53 +15.05)= 0 %
| Element | % | Atomic weight | Relative no. of atoms | Simplest Ratio |
| C | 77.42 | 12 | 77.42/12=6.45 | 6.45/1.07=6 |
| H | 7.53 | 1 | 7.53/1=7.53 | 7.53/1.07= 7 |
| N | 15.05 | 14 | 15.05/14= 1.07 | 1.07/1.07 =1 |
C:H:N=6 : 7: 1
Empirical formula =C6H7N
Empirical formula weight =12 x 6 +1 x 7 +14 = 93
Calculation of molecular weight by Platini chloride method-
2E+410/195 =W/w
w=0.195 gm.
W =0.596 gm.
2E+410/195 = 0.596 /0.195
=3.05
2E =(195 x 3.05) -410
E = 184.75 /2 = 92.38
E = 92.38
molecular weight =E x acidity of base
=92.38 x 1 = 92.38
n= molecular weight/ Empirical formula weight
n= 92.38 /93 = 0.99 =1.0
mol. formula =( Empirical formula )n
=(C6H7N)n
=(C6H7N)1
mol. formula = C6H7N Ans.
Q 3– 0.20 gm of a monobasic acid on combustion give 0.505 gm CO2 & 0.0892 gm. H2O. 0.183 gm. of this acid requires 15 ml of N/10 NaOH for complete neutralisation. Determine molecular formula.
Ans. % of C =(12 x wt. of CO2 x 100) /(44 x wt. of org.compd)
= (12 x 0.505 x 100) /(44 x 0.20)
% of H =(2 x wt. of H2O x 100) /(18 x wt. of org.compd)
% of H =(2 x 0.0892 x 100) /(18 x wt. of 0.20)
=4.95 %
% of C =68.86%, % of H=4.95%
% of O = 100–(68.86+4.95)
=26.19
| Element | % | Atomic weight | Relative no. of atoms | Simple Ratio | Simplest ratio |
| C | 68.86 | 12 | 68.86/12= 5.74 | 5.74/1.64=3.5 | 3.5 x 2=7 |
| H | 4.95 | 1 | 4.95/1= 4.95 | 4.95/1.64=3 | 3 x 2=6 |
| O | 26.19 | 16 | 26.19/16=1.64 | 1.64/1.64=1 | 1 x 2 =2 |
C : H : O=7 : 6 : 2
Empirical formula = C7H6O2
Empirical formula weight = 12 x 7 +6 x 1 +2 x 16 = 122
molecular formula =( C7H6O2 )n
Calculation of Molecular weight by volumetric method-
w/E =N V(l)
w=0.183 gm.,N =1/10 , V =15 ml. =.015 l
0.183 /E = .015/10
E= 122
Mol. wt. = E x basicity of acid = 122 x 1 =122
n= molecular weight / Empirical formula weight
n =122/122 =1
Mol. formula = (C7H6O2)1
molecular formula =C7H6O2 Ans.