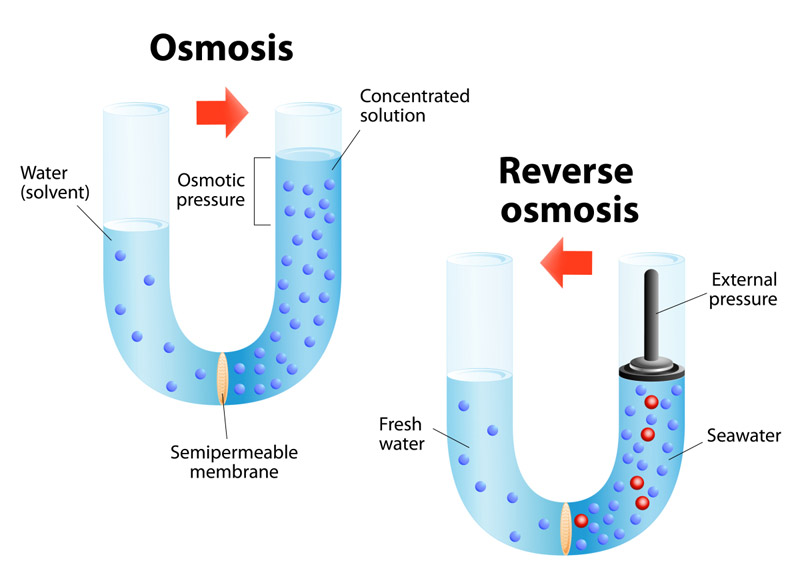
Osmosis-

source : Seven Days per Week
Osmosis-
This phenomenon is first observed by Abbe Nollet in 1748 & termed as osmosis ( means to push )

source : Saptarshi classes
‘It is defined as the spontaneous flow of solvent molecules through Semipermeable membrane from a pure solvent to the solution or from dilute solution to concentrated solution .’

source :Wikipedia
Diffusion –
‘In this process solute molecules move from concentrated solution to dilute solution and solvent molecules from dilute solution
to concentrated solution. This bilateral flow continues till the concentrations of both solutions become same . In this process no Semipermeable membrane is required . This phenomenon is known as diffusion.’

source : Quora
Semipermeable membrane (SPM) –
‘Those membranes which allow to pass only solvent molecules through them, are called Semipermeable membrane .’
Ex- Egg membrane , goat’s bladder, cell membrane , artificial membranes [ it is the film of gelatinous precipitates of some inorganic substances like Ca3 (PO4)2, Copper ferrocyanide ]

source : phys.org
Osmotic pressure-
‘The external pressure or excess pressure which must be applied on the solution to stop the flow of solvent into the solution through Semipermeable membrane is called Osmotic pressure.’
OR
‘The hydrostatic pressure developed as a result of osmosis is a measure of Osmotic pressure of the solution.’

source : Lumen Learning
Osmotic Pressure- a colligative property-
According to Vant Hoff , a dilute solution or ideal solution behaves like an ideal gas and different gas laws are applicable to the dilute solutions .
For dilute solution ,
Osmotic pressure (π) = CST
C = molar concentration of solution, T = temperature, S = gas constant
For a dilute solution , at a given temperature S and T are constant . So,
π = C
” Since Osmotic pressure depends upon the molar concentration of solution so , it is a colligative property”
Osmotic pressure (π) = CST
C= n / V = no. of moles / volume of solution in litres
π = n S T/ V
n = w /m = weight of solute / molecular weight of solute
π = w S T / m V
Unit of osmotic pressure-
π atm dyne /cm2 N/m2
V litre cm3 m3
S 0.0821 Litre atm K-1 mole-1 8.314 x 107 erg K-1 mole-1 8.314 J K-1 mole-1
C mole / litre mole / cm3 mole / m3
1 Litre = 1000 cm3 = 10 -3 m3