
Le-chatelier’s Principle
Le-chatelier’s Principle source : Grade 12uchem.weebly.com Le-chatelier’s Principle A/c to this principle “If a system is subjected to Continue Reading »
Equilibrium constant
Equilibrium constant source : socratic . org Equilibrium constant Consider a reversible reacn A +B <——> C+D Eq. Constt. Continue Reading »
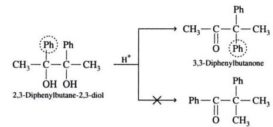
Pinacol-Pinacolone Rearrangement
Pinacol-Pinacolone Rearrangement source : you tube.com Conversion of 1,2-glycols(pinacol) to ketone or aldehyde(pinacolone) by means of acid is called Continue Reading »

Balance Redox Rxn by oxidation No. method
Redox Reaction source : credit-help.biz I2+NaOH → NaI+NaIO3 + H2O I20 → NaI–1 (Reduction) (i) (Decrease in O NO. Continue Reading »
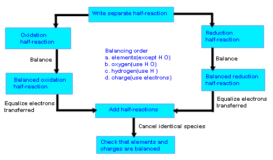
Balance Redox Equation by Ion-electron (Acidic medium)
Redox-reaction source:chemistry.tutorvista.com Balance the Given redox-reaction by ion-electron Method Cr2O7– – +Fe+++ H+ —-> Cr3++Fe3++H2O +6 Cr2O7– – + Continue Reading »

Practical Viva
practical viva source : www.rnib.org.uk Question1.What is Radical ? Ans. It is a group of atoms of one or Continue Reading »

Oxidation number solved problems
oxidation number Calculate Oxidation number of Underlined element- i) K2CrO4 ii) K2Cr2O7 2(+1)+x+4(-2)=0 2(+1)+2x+7(-2)=0 +2+x–8=0 +2+2x–14 =0 x=+6 Continue Reading »
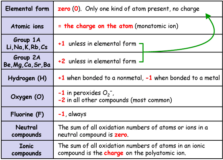
Oxidation Number – rules for calculation
oxidation number source : compound chem.com Oxidation number of an element in a particular compound Continue Reading »
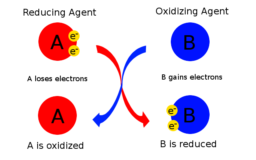
Oxidising & Reducing Agents
oxidising & reducing agents source : slide player.com Oxidising agents or oxidants : are substances which Show gain of Continue Reading »

oxidation & Reduction
oxidation & reduction source : www.layers-of-learning.com Oxidation Oxidation or de-electronation is a process which releases electrons. In oxidation, oxidation Continue Reading »