Question – Rank the following atoms and molecules based on which would effuse the fastest.
i) H2
ii) He
iii) N2
iv) CO2
v) H2S
Solution –
Molecular mass of H2 = 1 x 2 = 2
Molecular mass of He = 4
Molecular mass of N2 = 2 x 14 = 28
Molecular mass of CO2 = 12+ 16 = 44
Molecular mass of H2S = 2 x 1 + 32 = 34
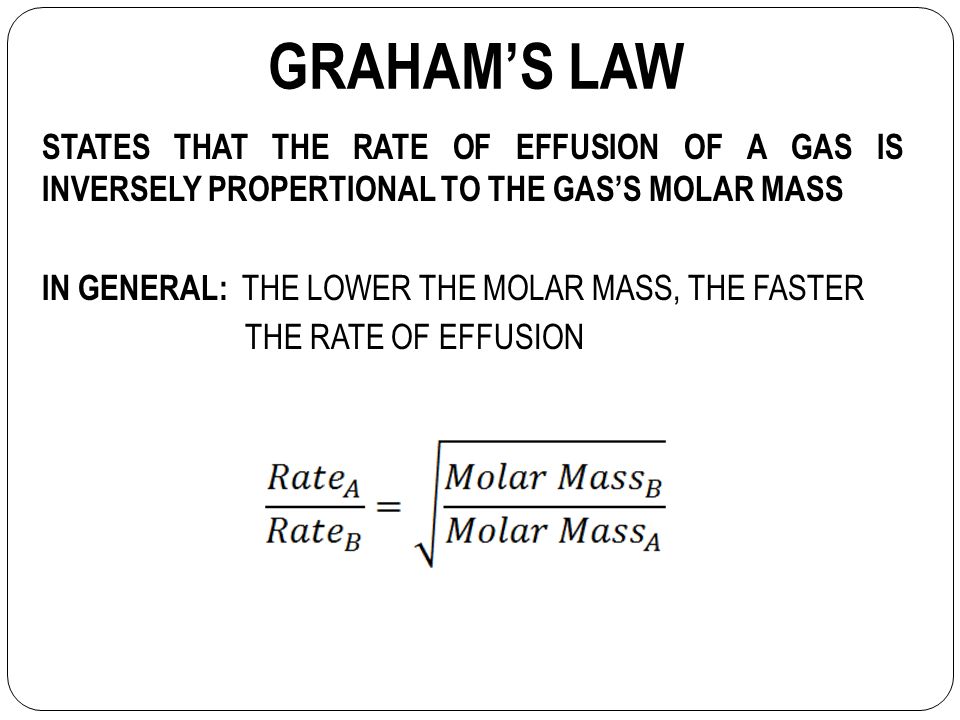
Effusion of gases also depends upon their molecular mass at constant temperature and pressure. According to Graham’s law ,
r1 / r2 = √ M2/M1
r ∝ 1 / √ M
Hence gas with lowest molecular mass will effuse faster.
Decreasing order of molecular mass of gases is,
CO2 , H2S, N2 , He , H2
Decreasing order of rate of effusion ,
H2 , He , N2 , H2S , CO2
H2 will effuse fastest and Co2 will effuse slowest.