Stannous Chloride

source : world of chemicals.com
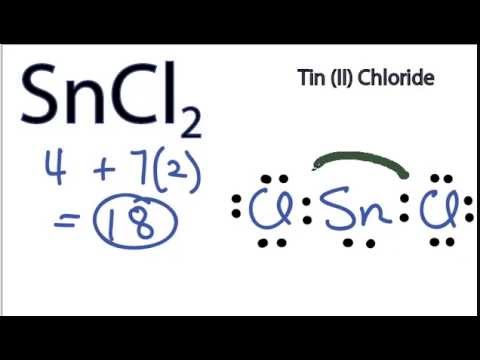
Stannous Chloride (SnCl2)
Method of preparation of anhydrous stannous chloride :
i) By heating tin metal in the presence of dry HCl gas :
Sn + 2HCl ———–> SnCl2 (Stannous chloride) + H2
ii) By heating Sn with mercuric chloride (HgCl2) :
Sn + 2HgCl2 ———> SnCl2 + Hg2Cl2 (mercurous chloride)
Properties :
1) It is a colourless, crystalline solid & soluble in water.
2) Reaction with NaOH : With small amount of sodium hydroxide , white ppt. of stannous hydroxide is obtained . Which is soluble in excess of sodium hydroxide due to formation of sodium stannite.
SnCl2 + 2NaOH ———> Sn(OH)2 (stannous hydroxide) + 2NaCl
Sn(OH)2 + 2NaOH ———-> Na2SnO2 (sodium stannite) + 2H2O
3) Reaction with H2S :
Brown precipitate of stannous sulphide is obtained which is soluble in yellow ammonium sulphide.
SnCl2 + H2S ———-> SnS + 2HCl
SnS + (NH4)2S2 ———-> (NH4)2SnS3 (soluble compound)
4) Effect of heat :
Tin oxy chloride is obtained
SnCl2 . 2H2O ———> Sn(OH)Cl ( Tin oxy chloride) + HCl + H2O
5) Reducing Properties :
Stannous chloride is a strong reducing agent. Itself it get reduced to Stannic chloride
a) Iodine is reduced to Hydrogen iodide –
SnCl2 + 2HCl + I2 ———> SnCl4 + 2HI
b) Mercuric chloride is reduced to white precipitate of mercurous chloride which is further reduced to grey precipitate of mercury.
SnCl2 + 2HgCl2 ———–> SnCl4 + Hg2Cl2 (mercurous chloride)
Hg2Cl2 + SnCl2 ———-> 2Hg (mercury) + SnCl4
c) Ferric chloride is reduced to ferrous chloride :
SnCl2 + 2FeCl3 ———> SnCl4 + 2FeCl2 (ferrous chloride)
d) Auric chloride is reduced to gold :
AuCl3 + SnCl2 ———-> Au (gold) + SnCl2
e) Cupric chloride is reduced to cuprous chloride :
SnCl2 + 2CuCl2 ———> 2CuCl (cuprous chloride) + SnCl4
Uses:
i) It is used as mordant in dye.
ii) In making colloidal solution of gold.
iii) As a strong reducing agent.
Conversions :
1) Tin to Anhydrous Stannous Chloride
Sn + 2HCl ———–> SnCl2 + H2
2) Stannous Chloride to Tin
SnCl2 + Zn (zinc metal) ——>Sn + ZnCl2