Applications of Solubility product
source : genchem.rutgers.edu
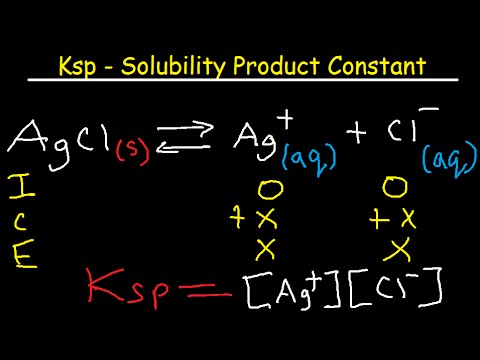
Applications of Solubility product-
i) Salting out of soap –
The important applications of Solubility product is Salting out of soap. Soap is sodium salt of higher fatty acid . It is precipitated from the solution by adding concentrated solution of NaCl( sodium chloride) .
C15H31COONa ————–> C15H31COO – + Na+
sodium palmitate
(soap)
NaCl ————> Na+ + Cl–
NaCl is strong electrolyte , it ionises completely in solution then concentration of sodium ions (Na+) increases.
Ionic product of [ C15H31COO –] [Na+] > Ksp of C15H31COONa (soap)
Hence soap precipitates out from the solution.
ii) Purification of common salt-
Natural common salt contains soluble and insoluble impurities . When saturated solution of common salt is prepared then insoluble impurities are filtered off.
HCl gas is passed in saturated solution of NaCl.
NaCl ———> Na + + Cl–
HCl ———> H + + Cl–
Both gives chloride ions( Cl–)in solution therefore concentration of Cl– increases. Hence,
ionic product of [Na +] [ Cl–] > Ksp of NaCl
Therefore pure NaCl precipitates out from the solution.
iii) Precipitation of IIIrd group metal hydroxides –
IIIrd group metal hydroxides [ Al(OH)3 , Fe(OH)3 , Cr(OH)3 ] get precipitated with ammonium hydroxide (NH4OH) in presence of ammonium chloride (NH4Cl).
NH4Cl ——–> NH4 + + Cl–
NH4OH ⇌ NH4+ + OH–
Because in presence of NH4Cl ( it is strong electrolyte and gives NH4 + ), dissociation of weak electrolyte NH4OH decreases due to common ion effect. Thus the concentration of [OH–] must be lowered. Thus
Ionic product of [ M+++] [OH–] 3 > Ksp of IIIrd group metal hydroxides
Thus only IIIrd group metal hydroxides get precipitated in IIIrd group.