
Heat of combustion & formation
Heat of combustion & formation- Heat of combustion – “It is defined as the change in internal energy Continue Reading »
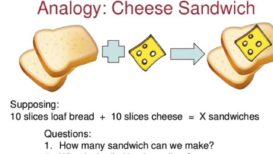
Stoichiometry numerical -2
Stoichiometry numerical Stoichiometry numerical- Question 1) How many moles of N2 are needed to produce 8.2 moles of NH3 Continue Reading »
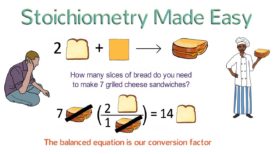
Stoichiometry numerical -1
Stoichiometry numerical- Question- 23 gm of sodium reacts with water. Calculate (i) the weight of H2 liberated (ii) the Continue Reading »

Stoichiometry
Stoichiometry Stoichiometry- In a reaction, quantitative relationships among the reactants & products is called ‘Stoichiometry’. Stoicheion means element & Continue Reading »

Thermodynamics
Thermodynamics- Thermodynamics – ‘The branch of science that deals with the study of different forms of energy & their Continue Reading »
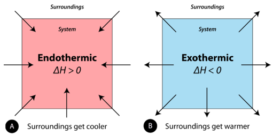
Enthalpy change
Enthalpy change- Enthalpy change- It is defined as the change in enthalpy or change in internal energy or amount Continue Reading »
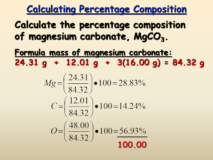
Percentage composition
Percentage composition- Percentage composition gives the mass of each element expressed as the percentage of the total mass. It Continue Reading »
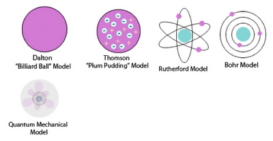
Dalton atomic theory
Dalton atomic theory To provide justification to the laws of chemical combination, Dalton proposed atomic theory. The basic postulates Continue Reading »
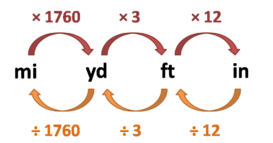
Unit conversion
Unit conversion- Unit conversion – One unit can be converted into another by conversion factor method or dimensional analysis. Continue Reading »

Properties of matter
Properties of matter- Properties of matter- Each substance has unique properties, these are physical & chemical properties. Physical properties Continue Reading »