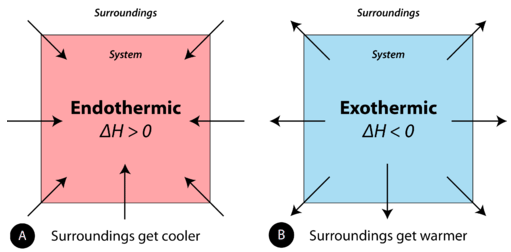
Enthalpy change-

wps.prenhall.com
Enthalpy change-
It is defined as the change in enthalpy or change in internal energy or amount of heat absorbed or released during the complete reaction as represented by a balanced thermochemical equation.
Ex- C + O2 —> CO2 ΔH = -94.3 Kcal
It means 12 gm of carbon reacts with 32 gm of oxygen to form 44 gm of carbon dioxide with the evolution of 94.3 Kcal of heat.
Factors influencing the Enthalpy change-
i) Physical nature of reactant & product-
For a given reaction the Heat of reaction varies with the change in the physical nature of reactants & products.
Ex- C(diamond) + O2. ———> CO2 ΔH = -94.3 Kcal
C(amorphous) + O2. ———> CO2 ΔH = -97.7 Kcal
It is clear from the example that the heat of reactions are different when two forms of carbon are different.
When product formed has a different physical state, then heats of reaction are also different.
H2 + 1/2O2 —-> H2O(l) ΔH = -68.3 Kcal
H2 + 1/2O2 —-> H2O(g) ΔH = -57.0 Kcal
ii) The Reaction carried out at constant pressure or constant volume-
Heat changes at constant pressure are expressed in terms of ΔH.
From the first law of thermodynamics,
At constant pressure, Qp = ΔH
Heat changes at constant volume are expressed in terms of ΔU.
From the first law of thermodynamics,
At constant pressure, Qv = ΔU
ΔH & ΔU are related by the formula,
ΔH= ΔU + PΔV
P is the pressure at which reaction is carried out & ΔV is the change in volume during the reaction.
Because, PΔV = Δn RT
Δn= no. of moles of gaseous product – no. of moles of gaseous reactant
Thus,
ΔH= ΔU + Δn RT
iii) Temperature-
The Heat of reaction also depends upon the temperature. ΔH varies with temperature due to variation in heat capacity of the system with temperature.
ΔH2 – ΔH1= ΔCp( T2-T1)
ΔH= ΔCp.ΔT
where H2 & H1 are change in enthalpy at temperature T2 & T1.
ΔCp = Cp of oroducts – Cp of reactants
ΔU2 – ΔU1= ΔCv( T2-T1)
ΔU= ΔCv.ΔT
where U2 & U1 are change in internal energy at temperature T2 & T1.
ΔCv = Cv of products – Cv of reactants