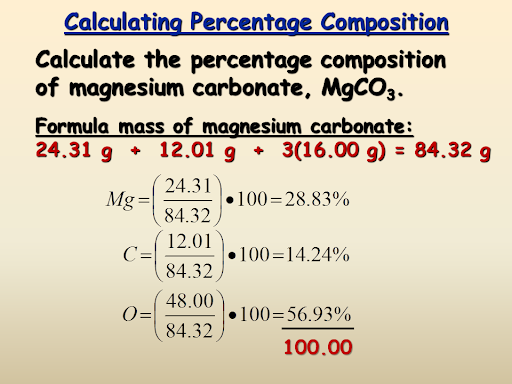
Percentage composition-

YouTube.com
Percentage composition gives the mass of each element expressed as the percentage of the total mass. It means it gives the number of grams of the element present in 100 gm of the compound.
Mass percentage of an element = mass of that element in 1 mole compound x 100 / Molar mass of the compound
Ex 1) Calculate the mass percentage composition of water(H2O).
Solution –
Molar mass 0f water = 2×1 + 16 = 18
Mass % of an element = mass of that element in 1 mole compound x 100 / Molar mass of the compound
Mass % of hydrogen = 2 x 100 / 18
Mass % of hydrogen =11.11 %
Mass % of oxygen = 16 x 100 / 18
Mass % of oxygen =88.89%
Ex 2) Calculate the mass percentage composition of sucrose (C12H22O11).
Solution –
Molar mass 0f sucrose = 12 x 12 + 22×1 + 11 x 16= 342
Mass % of an element = mass of that element in 1 mole compound x 100 / Molar mass of the compound
Mass % of Carbon = 144 x 100 / 342
Mass % of carbon = 42.11 %
Mass % of hydrogen = 22 x 100 / 342
Mass % of hydrogen = 6.43 %
Mass % of oxygen = 176 x 100 / 342
Mass % of oxygen =51.46%
Ex 3) Calculate the mass percentage composition of urea (NH2CONH2).
Solution –
Molar mass 0f urea(NH2CONH2 or N2H4CO)= 14×2 + 4×1 + 12 + 16= 60
Mass % of an element = mass of that element in 1 mole compound x 100 / Molar mass of the compound
Mass % of nitrogen = 28 x 100 / 60
Mass % of nitrogen =46.67 %
Mass % of Carbon = 12 x 100 / 60
Mass % of carbon = 20 %
Mass % of hydrogen = 4 x 100 / 60
Mass % of hydrogen =6.67 %
Mass % of oxygen = 16 x 100 / 60
Mass % of oxygen = 26.67 %
Ex 4) Calculate the mass percentage composition of ethyl alcohol or ethanol (C2H5OH).
Solution –
Molar mass 0f ethyl alcohol = 2 x 12 + 6×1 + 1 x 16= 46
Mass % of an element = mass of that element in 1 mole compound x 100 / Molar mass of the compound
Mass % of Carbon =24 x 100 / 46
Mass % of carbon = 52.17 %
Mass % of hydrogen = 6 x 100 / 46
Mass % of hydrogen = 13.04 %
Mass % of oxygen = 16 x 100 / 46
Mass % of oxygen = 34.78 %
Ex 5) Calculate the mass percentage composition of Copper pyrite (CuFeS2)
Solution –
Molar mass 0f Copper pyrite =63.5 + 56 + 2 x 32 = 183.5
Mass % of an element = mass of that element in 1 mole compound x 100 / Molar mass of the compound
Mass % of Copper = 63.5 x 100 / 183.5
Mass % of Copper = 34.60 %
Mass % of Iron = 56 x 100 / 183.5
Mass % of iron = 30.52 %
Mass % of Sulphur = 64 x 100 / 183.5
Mass % of sulphur = 34.88 %