Le-chatelier’s Principle

source : Grade 12uchem.weebly.com
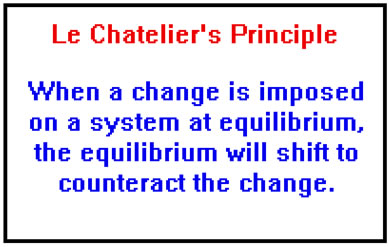
Le-chatelier’s Principle
A/c to this principle “If a system is subjected to a change of concentration , pressure or temperature , then the equilibrium shifts in the direction that tends to undo the effect of this change”
- If temperature is increased then equilibrium will shift in that direction where absorption of heat takes place. At low temperature equilibrium will shift in the direction where evolution of heat takes place.
- If pressure is increased, then equilibrium will shift in that direction where volume is decreased
- If concentration of reactants are increased then equilibrium shifts in forward direction. When concentration of products are increased then equilibrium Shifts in backward direction
Applications of Le-Chatelier’s Principle to physical Equilibria-
- Effect of temperature on solubility-
Most solids dissolve in H2O with the absorption of heat (endothermic reaction)
KNO3(s)+ water —-> KNO3(aq.) – Q K.cal
A/c to Le-chatelier’s principle
If temperature is raised then equilibrium will shift in the direction where this heat is used up. In forward reaction, heat is absorbed & equilibrium will shift in forward direction. Hence solubility of KNO3 will increase.
Ex. glucose , urea, Sugar, NaCl
Some solids evolve heat on dissolution in H2O (exothermic)
KOH(s) + water —-> KOH (aq.) + heat
If temperature is raised the equilibrium will shift in the direction where the absorption of heat takes place.
Ex. NaOH, CaO, Ca-Acetate
- Effect of Pressure on Solubility of gas
A/C to Le-chatelier’s Principle
At low pressure equilibrium will shift in backward direction while at higher pressure, equilibrium will shift in forward direction
Solubility of gas increases at higher pressure & decreases when pressure is lowered
- Melting of Ice-
Temperature– When ice melts, heat is absorbed & volume is decreased
When heat is given then equilibrium will shift in the direction where heat is absorbed. Hence equilibrium will shift in forward direction & ice melts.
Pressure- When pressure is increased, the volume decreases. The equilibrium will shift in the direction where volume is decreased.
Volume of water is less than ice therefore, at high pressure equilibrium will shift in forward direction & ice will melt into H2O
Applications of Le-Chatelier’s Principle to Chemical Equilibria
Ex. 1 Formation of HI-
H2 + I2 <——–> 2HI
A/c to Lechatelier’s principle
- Effect of Concn– If concn of any substance is increased in equilibrium mixture then equilibrium is shifted in that direction where consumption of that substance takes place.
If concentration of H2 or I2 is increased, then equilibrium shifts in the forward direction & concentration of HI increases.
- Effect of Pressure- If pressure is increased then equilibrium will shift in the direction where volume is decreased.
When number of moles on either side of a equation is the same, then there is no effect of change of pressure on equilibrium
- Effect of Temperature
H2 + I2 <——–>2HI + Q K.Cal
If temperature is increased then equilibrium will shift in that direction in which absorption of heat take place.
Formation of HI is exothermic therefore low temp favours the forward reacn & equilibrium shifts in forward direction
Favourable Condition
- i) High concentration of H2 or I2 or both (ii) Low Temperature
II Dissociation of PCl5
PCl5 <——>PCl3+Cl3 – Q k cal
Favourable Condition
1- High concentration of PCl5 (2) High temperature (3) Low pressure
III) Formation of NH3
N2 +3H2<——> 2NH3 +Q kcal
Favourable Condition
(1) High concentration of N2 or H2 or both (2) Low temperature (3) High pressure
IV) Formation of SO3
2SO2 +O2<——> 2SO3
Favourable Condition
(1) High concentration of SO2 or O2 or both (2) (3) High pressure
Effect of addition of Inert Gas-
- a) Addition of inert gas to an equilibrium (if ) has no effect
- b) If
- i) At const vol.- I addition is made at constt vol. them no. effect
- ii) At constt press. Addition of gas at constt pressure will favour the direction of rxn number total no. of moles at equili, show an increase.
- What is the effect of adding inst. gas
(i) (ii)
(iii) (iv)
(v)