Amine

source : silver bullet.in
Properties
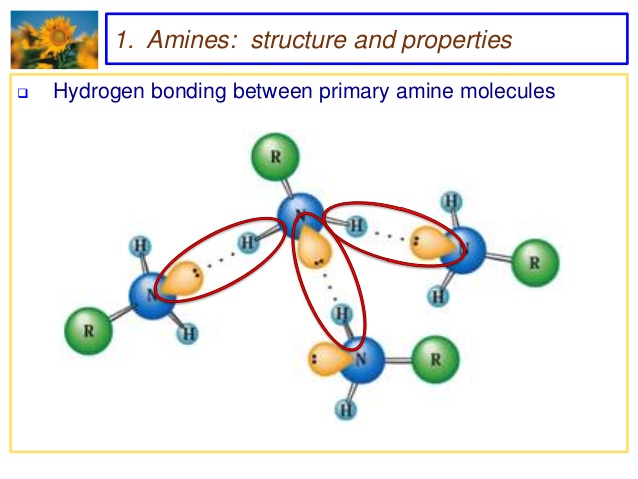
1) It is highly soluble in water due to formation of inter molecular hydrogen bonding with water.
2) Its aqueous solution turns red litmus blue because its aqueous solution is basic in nature.
3) Basic Nature :
Due to presence of lone pair of electron on Nitrogen atom, ethyl amine behaves like base. It is weak mono acidic base.
a) Reaction with H2O : It dissolves in water forming ethyl ammonium hydroxide.
C2H5NH2 + H2O ——> C2H5NH3OH
b) Reaction with Acids : It reacts with acids to form salt.
C2H5NH2 + HCl ——> C2H5NH2 .HCl or [C2H5NH3]+Cl- (ethyl ammonium chloride)
C2H5NH2 + H2SO4 —->[C2H2NH3]2SO4 (ethyl ammonium sulphate)
4) Reaction with Chloroplatinic Acid :
Ethyl amine platinichloride is formed.
2C2H5NH2 + H2PtCl6 —-> (C2H5NH2 )2 H2PtCl6
5) Reaction with Metal Salts :
Aqueous solution of ethylamine reacts with Aluminium , Chromium & iron salts , then metal hydroxides get precipitated.
FeCl3 + 3 C2H5NH3OH —-> Fe(OH)3 (Ferric hydroxide (brown ppt.) ) + C2H5NH3Cl
6) Reaction with Acetyl chloride or Acetic Anhydride :
N-ethyl acetamide or Acetyl ethylamine is formed.
C2H5NH2 +CH3COCl —-> CH3CONHC2H5 +HCl
C2H5NH2 + (CH3CO)2O —-> CH3CONHC2H5 (N – ethyl acetamide) + CH3COOH
7) Reaction with Ethyl halide :
A mixture of secondary , tertiary amine & Quaternary ammonium salt is formed.
C2H5NH2 + C2H5Br —–> (C2H5)2NH +HBr
(C2H5)2NH + C2H5Br——> (C2H5)3N +HBr
(C2H5)3N + C2H5Br —-> [(C2H5)4N]+ Br- (tetra ethyl ammonium bromide)
8) Reaction with Hinsberg’s Reagent :
Hinsberg”s Reagent is benzene sulphonyl chloride when ethylamine reacts with benzene sulphonyl chloride then N-ethyl benzene sulphonamide is formed which is soluble in NaOH.
C6H5SO2Cl + C2H5NH2 —-> C6H5SO2NHC2H5 (N-Ethyl benzene sulphonamide)
———>C6H5SO2N NaC2H5 (sodium salt of N-Ethyl benzene sulphonamide)
Hinsberg’s Reagent is used in the separation of primary, secondary & tertiary amine from their mixture. Secondary amine also form same compound with Hinsberg’s Reagent which is insoluble in NaOH. Tertiary amine doesn’t react with Hinsberg’s Reagent.
9) Reaction with Phenyl isocyanate :
N-ethyl-N-phenyl urea is formed.
C6H5NCO + C2H5NH2 —–> C6H5NHCONHC2H5
phenyl isocyanate
10) Reaction with NaNO2+HCl or HNO2 : Ethyl alcohol, N2 & H2O formed
NaNO2+HCl—–> HNO2 +NaCl
C2H5NH2 + HNO2——> C2H5OH + N2 +H2O
11) Carbyl Amine reaction :
Ethyl amine reacts with chloroform (CHCl3) & Alcoholic KOH (KOH in ethanol) , then ethyl isocyanide is formed which is a bad smelling compound .
C2H5NH2 + CHCl3 +3KOH(alc.)—->C2H5NC (ethyl isocyanide)+3KCl +3H2O
12) Reaction with Sodium metal :
H2 gas is evolved.
C2H5NH2 +2Na—-> 2C2H5NHNa +H2
13) Reaction with Grignand Reagent : Alkane is formed
C2H5NH2 + RMgX ——> RH + C2H5NHMgX
C2H5NH2 + CH3MgBr——> CH4 + C2H5NHMgBr
14) Hofmann Mustard Oil reaction : Ethylamine reacts with Carbon di sulphide (CS2) & Mercuric chloride (HgCl2) to form ethyl isothiocyanate which has smell like mustard oil.
C2H5NH2 + CS2 + HgCl2 —–> C2H5N=C=S (ethyl isothiocyanate) +HgS +2HCl
(smell like mustard oil)
15) Reaction with Aldelydes : It reacts with aldehyde to give schiff’s base.
C2H5NH2 + CH3CHO ——-> C2H5N=CHCH3(Ethylidene ethyl amine or schiff’s base) +H2O
C2H5NH2 + C6H5CHO ——-> C2H5N=CHC6H5(Benzilidene ethyl amine or schiff’s base) +H2O
Test for Ethyl amine (Primary amine) :
i) Carbylamine Test or Isocyanide test :
When ethylamine (primary amine-aliphatic or aromatic) is heated with CHCl3 & Alc. KOH , then ethyl isocyanide is formed which is a bad smelling compound.
RNH2 + CHCl3 +3KOH(alc.)—->RNC (Alkyl isocyanide)+3KCl +3H2O
C2H5NH2 + CHCl3 +3KOH(alc.)—->C2H5NC (ethyl isocyanide)+3KCl +3H2O
ii) Hofmann Mustard Oil test : Ethylamine reacts with Carbon di sulphide (CS2) & Mercuric chloride (HgCl2) to form ethyl isothiocyanate which has smell like mustard oil.
iii) Nitrous Acid test:
When ethylamine reacts with HNO2, C2H5OH is formed which give yellow crystals of lodo form with I2 & NaOH
Read more articles at chemistryonline.guru