Applications Colloids

source : slide share.net
Applications Colloids
Few important applications of colloid are-
-
In Industry
Colloids are very effective catalysts because of their large surface area. Paint, varnishes, enamels, celluloses, resins, gums, glass & other athesives like nylon, terelene, rayon, leather, textile, paper etc.
-
Foods
Many of our foods are colloidal in nature food is easily digested when in the colloidal state. Milk is stabilized by casein which itself is a lyophillic colloid, Gelatin is added to ice -cream as a protective colloid, to preserve its smoothness. Other examples are -butter, cheese, fruit jelly, lassi, Halwa, Biscuits, Roti etc.
-
In Medicines
Many medicines are more effective in their colloidal form because in colloidal state they are easily assimilate & absorb. Antibiotics such as Penicillin & streptomycin are produced in colloidal form suitable for injection. Colloidal gold, manganese calcium are used in intramuscular injection for the treatment of tuberculosis & rickets. In plants, colloidal sulphur is used as germ killer. Argygrol & protargal are colloidal solution of silver & are used as eye medicine. Colloidal antimony is used in curing Kalazar.
Rubber-Planting
The negatively charged particles of rubber are made to deposit on the wires or handles of various tools.
In purification of water-
Impure water may contain dust & soil particles. These impurities are negatively charged colloidal particles. They are removed by addition of a small amount of alum. Alum contains Al+++. According to Hardy-Schulze rule it has high coagulating power. The dust & soil particles are coagulated & removed by filteration. so it is an important application of colloids
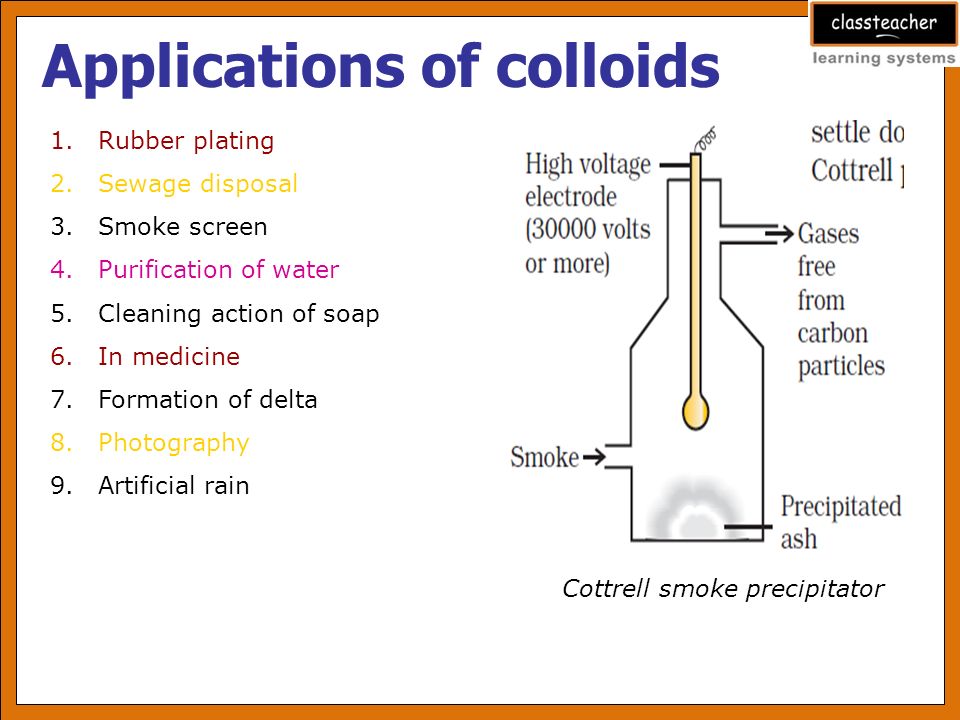
In Smokc Precipitation –
Smoke is a colloidal solution of small particles of carbon dispersed in air smoke is negative colloid. The excessive smoke coming from chimneys of industries, pollute the atmosphere. This health hazard can be checked by precipitating smoke by using positively charged cottrell. Negatively charged particles of carbon come in contact with cottrell & become discharged & precipitated. Thus clean air comes out of the chimney.
In clearing the clothes
Dirt or dust sticks on the grease of oil which gathers on cloth. Grease is not wetted by water & difficult to clean the garments by water alone. The addition of soap lowers the interfacial tension between water & grease. This causes the emulsification of grease in water. The mechanical action as rubbing, releases the dist.
Formation of Delta
A triangular pices of land (Called delta) is formed at a place where a river meets a sea. Formation of delta can be explained on the basis of the fact that the soil, sand & dist particles present in river water are negatively changed colloidal particles. When they come din contact with sea water containing minerals like NaCl. They are coagulated & a triangular piece of land (Called delta) in formed.
Sewage disposal-
The Sewage is colloidal in nature, where dirt & mud are dispersed in water.Therefore Sewage in big cities is passed through big tanks fitted with electrodes. Dirt & Mud particles are coagulated & settle down. The dirt deposited at the electrodes is removed & used as manure.
FOG
When a large mass of air, containing dust particles, is called belon its dew point then the moisture of air condenses on the surface of dust particles forming fine droplets. These droplets being colloidal in nature continue to float in the air in the form of fog.
Read more articles at chemistryonline.guru