Haber’s Process

source :chem-guide.blogspot.com
Haber’s Process:
Principle: NH3 is manufactured by the reaction of N2 and H2.
N2 + 3H2 <———> 2NH3 + 22.4 Kilo Cal. energy
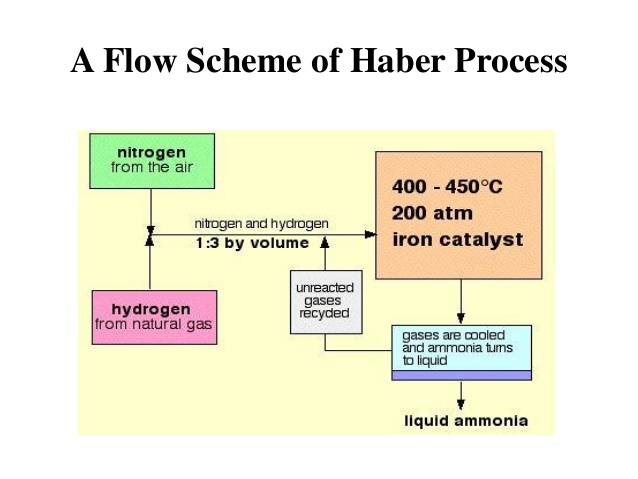
This reaction is reversible. Forward reaction is exothermic. According to Lechatelier’s principle high pressure and low temperature will favour the formation of NH3. Therefore pressure is kept at 200 oC. Since activation energy of reaction is very high so NH3 is not formed at low temperature. Catalyst is added to lower the activation energy and 500oC temperature is kept.
Thus 200 atmospheric pressure and 500 oC temperature is kept to manufacture NH3. Finely divided Fe acts as catalyst and Mo (or mixture of K2O + Al2O3 ) is used as promoter . The function of promoter is to increase the activity of catalyst.
N2 + 3H2 <—— > 2NH3 + 22.4 K. Calorie energy
Process :
Nitrogen is obtained by the fractional distillation of air and hydrogen obtained from water gas is mixed in the ratio of 1 : 3 and then compressed to 200 atmosphere pressure.
It is then passed through purifier and drying chamber. This chamber is filled with soda lime (NaOH + CaO). The impurities of CO, CO2, H2S, SO2, H2O etc are present in the gaseous mixture are removed. These impurities decrease or destroy the activity of the catalyst. So it is necessary to remove these impurities.
The pure & dry mixture N2 and H2 is passed through catalyst chamber. This chamber is filled with finely divided iron catalyst and Mo promoter. Temperature of the catalyst chamber is kept at 500oC. In this chamber N2 and H2 react to form NH3 and heat is produced. The heat produced maintains the temperature of the catalyst.
The gaseous mixture coming out from catalyst chamber contains about 10% NH3. The rest is N2 and H2. The gaseous mixture is cooled in water condenser where NH3 is liquefied and is collected and the remaining N2 and H2 is sent back to catalyst chamber with the help of circulation pump. This process continues and liquefied NH3 is obtained.