Salt hydrolysis

source: Worksheets Library
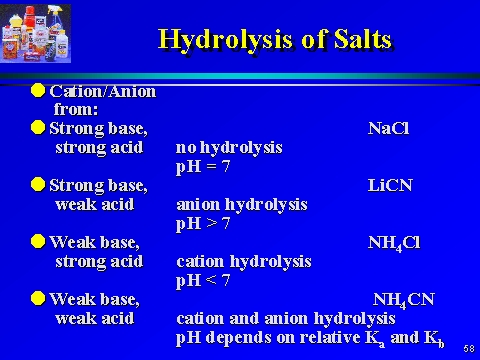
Salt hydrolysis-
Q 1) Calculate the degree of hydrolysis and pH of 0.1 M sodium acetate solution. Hydrolysis constant of CH3COONa is 5.6 x 10-10.
Solution )
Sodium acetate is a salt of weak acid and strong base.
On Salt hydrolysis-
CH3COO– + H2O ⇌ CH3COOH + OH-
weak ion
Before hydrolysis C 0 0
After hydrolysis C ( 1-h) Ch Ch
Kh = [CH3COOH][OH–] / [CH3COO–]
Kh = Ch x Ch / C (1-h)
Kh = Ch2/(1-h)
because, h <<<1 ,
So,
1-h =1
Kh = Ch2
C = 0.1 M , Kh = 5.26 x 10 -10
h = √ Kh / C
h = √ 5.6 x 10 -10 / 0.1
h = √ 56 x 10 -10
Degree of hydrolysis ‘h’ = 7.48 x 10 -5 Ans.
[OH–] = Ch =0.1 x 7.48 x 10 -5 = 7.48 x 10 -6 M
[H+ ] [OH–] = Kw
[H+] = Kw / [OH–] = 10 -14 / 7.48 x 10 -6
[H+] = 1.33 x 10 -9 M
pH = -log [H+]
= – log [1.33 x 10 -9]
= – [log 1.33 – 9 log 10]
= – log 1.33 + 9 log 10]
= – 0.124 +9=8.876
pH = 8.9 Ans.
Q 2) Calculate the hydrolysis constant for NH4Cl , pH and [OH–] in 0.1 M NH4Cl solution ?
(Kb or K NH4OH = 1.75 x 10-5 , Kw = 1 x 10-14)
Solution )
i)
Kh = Kw / Kb
Given, Kb= 1.75 x 10-5 , Kw = 1 x 10-14
Kh = 1 x 10-14 / 1.75 x 10-5
Hydrolysis constant ‘Kh‘ = 5.7 x 10 -10 Ans.
ii) Calculation for pH-
On Salt hydrolysis-
NH4 + + H2O ⇌ NH4OH + H +
weak ion
Before hydrolysis C 0 0
After hydrolysis C ( 1-h) Ch Ch
Kh = Ch x Ch /C ( 1-h) = Ch2 /(1-h)
h <<<1
So, (1-h) = 1
Kh = Ch2
C = 0.1 M
h = √ Kh / C
h = √ 5.7 x 10 -10 / 0.1
h = √ 57 x 10 -10
h = 7.55 x 10 -5 Ans.
[H+] = Ch = 0.1 x 7.55 x 10 -5
[H+ ] = 7.55 x 10 -6
pH = -log [H+] = – log (7.55 x 10 -6)
= – (log 7.55 – 6 log 10)
= – ( 0.8779 – 6 x 1)
= -0.8779 + 6
pH = 5.12 Ans.
Alternate method for calculation of pH-
NH4Cl is a salt of strong acid and weak base . Therefore ,
pH = 7 – 1/2 pKb – 1/2 log C
Kb =1.75 x 10 -5, C = 0.1 M
pKb = -log Kb= -log (1.75 x 10 -5)
= -(log 1.75 + log 10 -5)
= -(log 1.75 – 5 log 10 )
= – ( 0.245 – 5 x 1)
= – ( – 4.755)
pKb = 4.755
pH = 7 – (4.755/2) – 1/2 log 0.1
=7 – 2.377 – (-1/2)
= 7.5 – 2.377
pH = 5.123 Ans.
iii) Calculation for [OH–]
[H+][OH–] = 10-14
[OH–] = 10 -14 / 7.55 x 10-6
[OH–] = 1.32 x 10-9 M Ans.
Q 3) Calculate the pH of an aqueous solution of 1.0 M ammonium formate
( HCOONH4) assuming complete dissociation . pKa for HCOOH = 3.8 , pKb for NH4OH = 4.8
Solution )
Ammonium formate is a salt of weak acid and weak base.So,
pKa = 3.8 , pKb = 4.8
pH = 7 + 1/2 pKa – 1/2 pKb
= 7 + (3.8/2) – (4.8/2)
= 7 + 1.9 – 2.4 = 6.5
pH = 6.5 Ans.