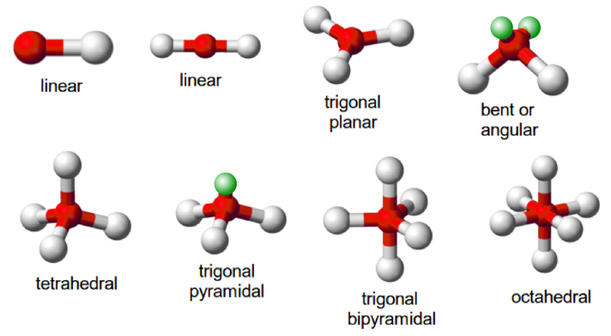
VSEPR Theory

source : justinbechthold.wordpress.com
VSEPR Theory [Valence Shell Electron Pair Repulsion Theory] -Applications-
A) Shapes of molecules containing bond pairs only-
i) Shape of BeCl2 molecule-
Be – atom is surrounded by two bond pair of electrons . Geometry of BeCl2 molecule is linear and bond angle is 180 0.
Other Ex.- BeF2 , ZnCl2 , HgCl2

source : Slide Share
ii) Shape of BCl3 molecule-
Valence shell of Boron has three valence electrons. So each electron of Boron form a bond pair with Cl – atom. Hence B- atom is surrounded by three bond pairs. Geometry of molecule is trigonal planar.
Other Ex.- BF3 , AlCl3
source : Study.com
iii) Shape of CH4 molecule-
Valence shell of Carbon has four valence electrons. So each electron of Carbon-atom form a bond pair with H – atom. Hence C- atom is surrounded by four bond pairs. Geometry of molecule is tetrahedral and bond angle is 109028′.
Other Ex.- SiF4 , CCl4 , SiH4, NH4+

source : revolvy.com
iv) Shape of PCl5 molecule-
Phosphorus has five valence electrons . These five valence electrons are bonded by 5 Cl -atoms forming 5 bond pairs around the P – atom.So geometry is trigonal bipyramidal . In PCl5 all the bond angles are not equal. The three bond pairs are in the same plane at an angle of 1200, while other two bond pairs are perpendicular to the plane, making an angle of 900 with the plane.
Ex . – PF5

source : Lumen Learning
v) Shape of SF6 molecule-
Six valence electrons are present in central Sulphur atom . Each of these six forms bond with F – atom. Geometry of molecule is octahedral and all bond angles are same (900).

source : Lumen Learning
vi) Shape of IF7 molecule-
Iodine has seven valence electrons . These seven valence electrons are bonded by seven F -atoms forming seven bond pairs around the I – atom . So geometry is pentagonal bipyramidal . In iodine hepta fluoride (IF7) all the bond angles are not equal. The five bond pairs are in the same plane at an angle of 720, while other two bond pairs are perpendicular to the plane, making an angle of 900 with the plane.

source : wikipedia