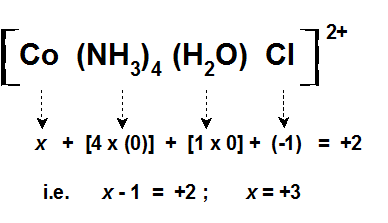Coordination compound
source : intranet.tdmu.edu.ua
Coordination compound : IUPAC name
Solved Examples:
1) K4[Fe (CN)6]
Oxidation number of Fe = x
4(+1) + x +6(-1) = 0
x= +2
Potassium hexacyano ferrate (II)
2) [Ag(NH3)2]Cl
Oxidation number of Ag = x
x +2(0) + 1( -1) =0
x =+1
Diamine Silver (I) chloride
3) [Cu(NH3)4]SO4
Oxidation number of Cu = x
x +4(0) +1( -2) =0
x =+2
Tetraamine copper (II) sulphate
4) Fe4[Fe(CN)6]3
Oxidation number of Fe = x
4( +3) +[x +6(-1) ]3 =0
12 +3x-18 =0
x =+2
Ferric hexa cyano ferrate (II)
5) [Co(NH3)6]Cl3
Oxidation number of Co = x
x +6(0) +3(-1) =0
x =+3
Hexa amine cobalt (III) chloride
6) [Co(NH3)5Cl]Cl2
Oxidation number of Co = x
x +5(0)+1( -1)+2(-1) =0
x =+3
Penta amine chloro Cobalt (III) chloride
7) K2[Ni(CN)4]
Oxidation number of Ni = x
2(+1)+x +4(-1) =0
+2 +x -4 =0
x =+2
Potassium tetra cyano nickelate (II)
8) Na[Ag(CN)2]
Oxidation number of Ag = x
+1+x +2(-1) =0
x =+1
Sodium dicyano argentate (I)
9) [Cu(NH3)2]Cl
Oxidation number of Cu = x
x +2 (0) +1 (-1 )=0
x =+1
Diamine Copper(I) chloride
10) Na4[Ni(CN)4]
Oxidation number of Ni = x
4(+1)+x +4(-1) =0
x =0
Sodium tetra cyano Nickelate (O)
11) Na3[Co(C2O4)3]
Oxidation number of Co = x
3(+1)+x +3(-2) =0
x =+3
Sodium trioxalato cobaltate(III)
12) K3[Fe(CN)6]
Oxidation number of Fe = x
3(+!) +x +6( -1) =0
x =+3
Potassium hexa cyano ferrate (III)
13) Ni[(CO)4]
Oxidation number of Ni = x
x +4(0) =0
x =0
Tetra carbonyl Nickel (0)
14) [Fe(CO)5]
Oxidation number of Fe = x
x +5(0) =0
x =0
Penta carbonyl Iron (0)
15) [CoCl (NH3)5]Cl2
Oxidation number of Co = x
x +1(-1) +5(0) +2(-1) =0
x =+3
Penta amine chloro Cobalt (III) chloride
16) [Co(NH3)4Cl2]Cl
Oxidation number of Co = x
x +4(0) +2(-1)+1( -1 ) =0
x =+3
Tetra amine dichloro Cobalt (III) chloride
17) [Co(NH3)5(H2O)] Cl3
Oxidation number of Co = x
x +5 x 0 + 1 x0 +3(-1) = 0
x= +3
Penta amine aqua Cobalt (III) Chloride
18) [Cr(en)2Cl2]+
x +2 x 0 +2 (-1) = +1
x =+3
Dichloro bis- (ethylenediamine) Chromium (III) ion
19) [Pt (NH3)2 Br2]
x + 2 x 0 +2 (-1) =0
x = +2
Diamine dibromo Platinum (II)
20) [Pt (NH3)4 Br2]2+
x + 4 x 0 +2 (-1) =+2
x = +4
Tetra amine dibromo Platinum (IV) ion
21) [Co(ONO) (NH3)5]Cl2
x + 1(-1) +5 x 0 +2 (-1) =0
x =+3
Penta amine nitrito Cobalt (III) Chloride
22) [Co(NCS) (NH3)5]Cl2
x + 1(-1) +5 x 0 +2 (-1) =0
x =+3
Penta amine isothiocyanato Cobalt (III) Chloride
23) [ Al (H2O)5 (OH)]2+
x + 5 x 0 +1 (-1) =+2
x = +3
Penta aqua hydroxo Aluminium (III) ion
24) [Cr (en)2 Cl2]+
x + 2 x 0 + 2 (-1) =+1
x = +3
Dichloro bis-(ethylenediamine) Chromium (III) ion
25 ) [Mo (en)2 (ONO)2]+
x + 2 x 0 +2 (-1) = +1
x = +3
Bis- (ethylenediamine) di nitrito Molybdenum (III) ion
26) K3[Fe (C2O4)3]
3(+1) +x +3(-2) =0
+3 + x -6 =0
x =+3
Potassium tri oxalato Ferrate (III)