RAOULT’S LAW
source : chemwiki.ucdavis.edu
RAOULT’S LAW
Lowering of vapour pressure-
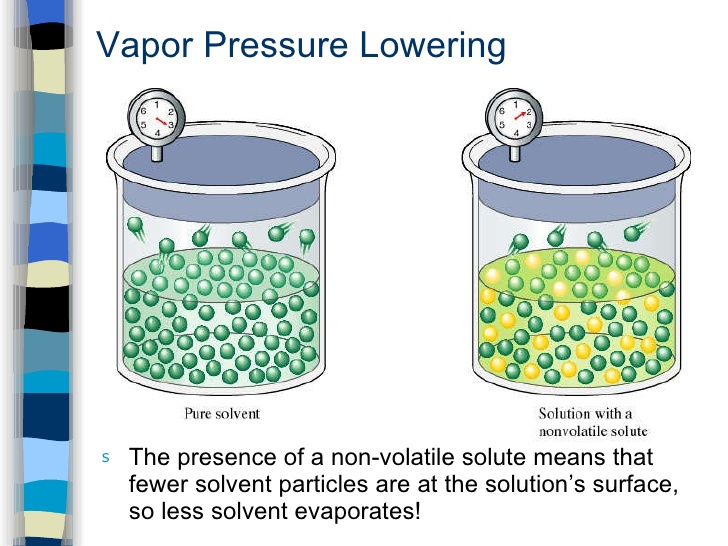
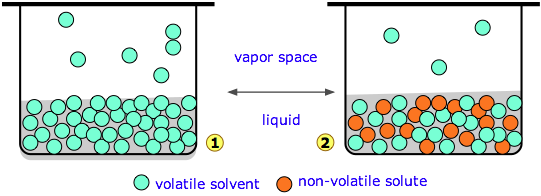
The molecules of liquid are in a state of constant motion in all directions, some of these molecules have higher kinetic energy than others. Some molecules which are present at the surface of a liquid may leave the surface & go into vapour state. The pressure exerted by the vapours of a liquid on its surface is known as vapour pressure of that liquid.
Vapour pressure of a liquid depends upon temperature & is independent of amount of liquid & vapours present in the system.
When a nonvolatile solute is dissolved in a liquid, the escaping tendency of liquid molecules is lowered hence vapour pressure of liquid is lowered. This difference of vapour pressure is called lowering of vapour pressure.
Lowering of vapour pressure= p-ps
p=vapour pressure of pure solvent
ps= vapour pressure of pure solution
Relative lowering of vapour pressure = p-ps/p
Raoult’s Law-
According to Raoult’s law,
“The relative lowering of vapour pressure of solvent is equal to the mole fraction of the solute.”
Let, No. of moles of solute = n
No. of moles of solvent = N
Total moles = n+N
Vapour pressure of pure solvent= p
Vapour pressure of solution =Ps
lowering of vapour pressure= p-ps
Relative lowering of vapour pressure= p- ps/p
So, According to Raoult’s Law,
p- ps/p =n / n+N
Limitation of Raoult’s Law-
Raoult’s law is followed by dilute solutions
1- The mole fraction of the solute in a given solution remains constant at all temperatures
2- Lowering of vapour pressure is a colligative property.
For solute that dissociates or associated in solution, Vant Hoff factor (i) is defined as-
Vant Hoff factor (i) = no.of solute particles after dissociation or association / no. of solute particles dissolved
The term ‘n’ is replaced by ( i * n).
- Molecular weight of solute can be calculated by using Raoult’s law as follows-
p- ps/p =n / n+N
n =w/m ; N =W/M
p /p-ps =n+N/n
(p /p-ps) -1 = (n+N/n ) -1
ps/p-ps =N/n
p-ps /ps =n/N
n=w/m : N =W/M
p-ps /ps =wM/mW
m =w. M.ps /W(p-ps)
When dissociation or association takes peace, then ‘n’ is replaced by i × n
m=i.w.M.ps /W(p-ps)
- Consider a solution of two liquids A & B, their vapour pressures in pure state be p1 & p2, their vapour pressures in solution be ps1 & ps2, their no. of moles in solution be n1 & n2.
According to Raoult’s law
p- ps/p =n / n+N
1 – (ps /p) =n / n+N
ps/p =1 – (n / n+N )
ps/p =N/n+N
ps = p (N/n+N )
Vapour pressure of solution = vapour pressure of pure solvent × mole fraction of solvent
ps1 = p1 ( n1/n1+n2)
ps2 = p2 ( n2/n1+n2)
Vapour pressure of solution = ps1+ps2
= p1 [ n1/(n1+n2)] + p2[ n2/(n1+n2)]
= ( p1.n1 + p2.n2) /(n1+n2)
= p1 [n1 +( p2 .n2/p1)]/ (n1 + n2)
If p2 >p1, the vapour pressure of solution will be greater than the vapour pressure of A & lesser than vapour pressure of B.
If p2<p1, the vapour pressure of solution will be less than the vapour pressure of A & greater than the vapour pressure of B.