Acid-base Indicators : Theories –
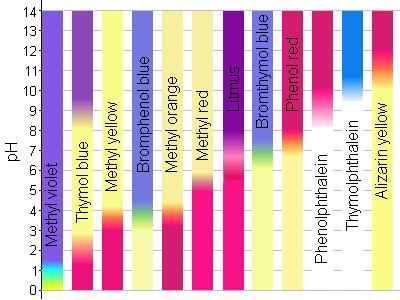
source : scottscience.weebly.com
Acid-base Indicators : Theories –
(1) Ostwald’s Theory-
According to this theory
- Acid-base indicators are weak organic acid or base
- They possess different colours in ionised & unionised state .
![]()
(Colourless) (Red Colour)
- The colour of the indicator depends upon the relative proportions of the unionised indicator molecules & its ions.
- Phenolphthalein is a weak acid whose unionised molecule is colourless while ion is red in colour.
![]()
![]()
(Colourless) (Colourless) (Red )
- Similarly Methyl orange is a weak base whose unionised molecules are yellow, while ions are red in colour
![]()
(Yellow) (Red) (Colourless)
- The ionisation of indicator is effected in presence of acid & base because
2- Modern Quinonoid theory-
According to this theory
- An acid- base indicator is dynamic equilibrium mixture of two tautomeric forms, one form is benzenoid while other is quinonoid.
- The two forms have different colours.
- Out of these one form exist in acidic soln, while the other in alkaline soln.
- The change of pH causes the conversion of benzenoid form into quinonoid form & vice versa & consequently colour changes.
- Benzenoid form is colourless or light in colour while the colour of quinonoid form is dark.
Ex. : Phenolphthalein :
HPh has benzenoid form in acidic medium which is colourless while it has quinonoid form in alkaline medium which has pink colour.

Benzenoid -form Quinonoid -form
(colourless in acidic medium) (pink colour in alkaline medium)
Ex. Methyl Orange :
MeOH has quinonoid form in acidic medium & benzenoid form in alkaline medium. The colour of benzenoid form is yellow & quinonoid form is red.

Read more articles at chemistryonline.guru