universal gas constant

source : slideplayer.com
units of universal gas constant-
- Value of R in M.K.S. System
According to Avogadro’s law, the volume of one mole of a gas at NTP is 22.4 litre. Standard temperature & pressure at N.T.P. are 00C & one atmosphere
P=1 atm.
V=22.4 l
n= 1 mole.
T= 2730K
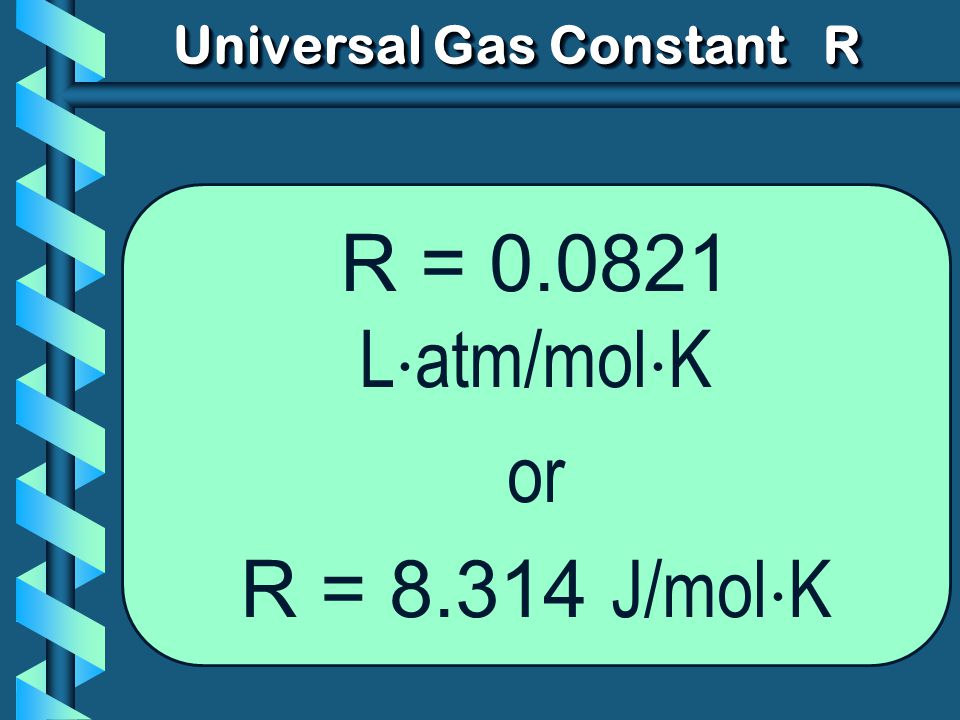
R=0.0821 litre atmosphere/degree/mole.
- Value of R. in C.G.S. System
If P is expressed in dyne/Cm2 & V in Cm3
P=1 atm = 76 cm. of Hg = 76×13.6×981 dyne/cm2
V= 22.4 litre = 22400 ml = 22400 cm3
n= 1 mole
T= 0+273 = 2730K
= 8.3 ×107 erg/degree/mole
= 8.3 Joule/degree/mole
Because one calorie is equivalent to 4.08×107 erg. So
R =[ 8.3×107 erg/degree/mole × 1calorie] /( 4.08×107 erg )
= 2 calorie/degree/mole