Activation energy-

source: The Biology Project – University of Arizona
Threshold Energy-
“The minimum amount of energy which must be associated with molecules so that mutual collisions may result in chemical reaction is termed as threshold energy .”
or
” The minimum amount of energy , which the colloiding molecule must possess as to make the chemical reaction to occur , is called threshold energy.”
The average kinetic energy of molecules is directly proportional to the absolute temperature . The energy of maximum molecules lies near the average value and few molecules have less or high value of kinetic energy. Effective collision is possible when molecules possess high energy. Thus some extra energy has to be supplied to the reactants to make their energy equal to threshold energy.
” The excess energy required by the reactants to undergo chemical reaction is called activation energy.”
or
” The excess energy that the reactants molecules having energy less than the threshold energy for reaction is known as activation energy.”
The activation energy is equal to the difference between the threshold energy needed for the reaction and the average kinetic energy of all the reacting molecules. It is denoted by Ea.
Activation energy = Threshold energy – energy possessed by molecules initially
Ea = E (threshold) – E (reactants)
Threshold energy = energy possessed by molecules initially + Activation energy

source : socratic
If Ea for a reaction is low , large number of molecules can have this energy and the fraction of effective collision will be large and reaction will proceed at high rate .
LOW ACTIVATION ENERGY ———> FAST REACTION
If Ea for a reaction is high , then fraction of effective collision will be small and reaction will proceed at low rate .
HIGH ACTIVATION ENERGY ———> SLOW REACTION
Progress of reaction –
The reactants do not change directly into the products. The reactants first absorb energy equal to the activation energy and form activated complex. At this state , molecules have energy equal to the threshold energy. It means the reaction involves some energy barrier which must be overcome before the products are formed. This energy barrier is known as ‘Activation energy barrier’.

source : www.digipac.ca
The concept of activation energy as applied to chemical reactions can be explained by plotting energy against progress of reaction.
A ⇌ B
The point ‘A’ represents the initial energy E1 of the reactants. ‘AB ‘ represents the energy barrier which the reactants have to cross. ‘C’ represents the energy of products i-e ‘ E2’.
In the starting of rising part of curve ‘AB’, the molecules come close to each other , but they can not react because they possess energy less than threshold energy. When they got sufficient energy , then they reach the ‘B ‘ point of energy barrier. This state is called activated state. At this state bonds between reacting molecules become feeble and probability of formation of new bonds between atoms of the molecules of reactant is strong. This probability is shown by curve ‘BC’ moving down the barrier .
If reactants possess higher total energy than the products , there will be release of energy.The reaction is exothermic. The heat evolved gives the heat of reaction.
Δ E = E1 – E2
An exothermic reaction has lower Ea and thus faster rate of reaction.
In case of backward reaction , energy of product is less than energy of reactants . This energy will be absorbed , the backward reaction is endothermic . An endothermic reaction has always greater Ea and has a slower rate of reaction than opposite reaction.
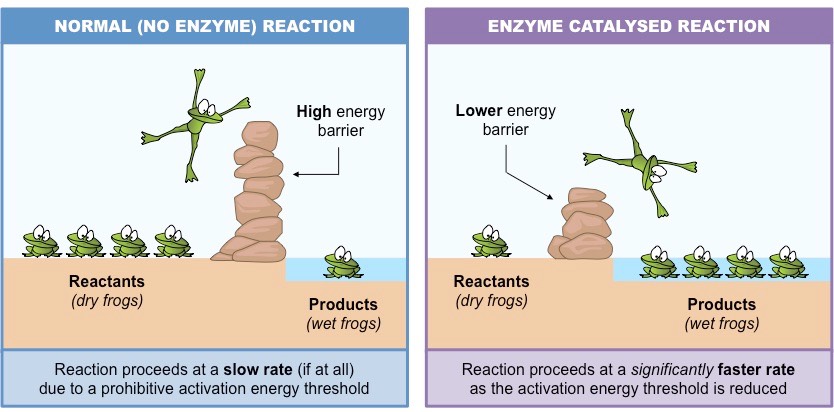
Effect of catalyst-
a) Presence of positive catalyst-

source : slideplayer.com
The function of a positive catalyst is to lower down the activation energy. In presence of a catalyst . the reaction follows a path of lower activation energy . As the energy barrier is reduced in magnitude, larger number of reacting molecules are able to cross over the energy barrier and thus rate of reaction increases.

source : fainaidea.com
b) Presence of negative catalyst-
A negative catalyst increases the activation energy of reaction by forming a new path of higher energy.Due to increased Ea , some active molecules become inactive , therefore rate of reaction decreases.