Alum

source : my article library.com
Alum
It is double sulphate having molecular formula M’2SO4 . R”’2(SO4)3 . 24H2O
Or
M’R”’ (SO4)2 . 12H2O

Example: K2SO4 . Al2 (SO4)3 . 24H2O(Potash alum)
K2SO4 . Cr2(SO4)3 . 24H2O (Chrome alum)
Potash alum (K2SO4 . Al2(SO4)3 . 24H20 :
Methods of preparation:
1) By potassium sulphate or Aluminium sulphate :
When equimolar amount of potassium sulphate or aluminium sulphate are dissolved in water then solution on concentration gives crystals of alum.
K2SO4 + 3Al2(SO4)3 + 24H2O ———> K2SO4 . Al2(SO4)3 . 24H2O
2) From Alunite :
K2SO4 . Al2(SO4)3 . 4Al(OH)3 + 6H2SO4 ———>K2SO4 + 3Al2(SO4)3 + 12H2O
K2SO4 + 3Al2(SO4)3 + 24H2O ———> K2SO4 . Al2(SO4)3 . 24H2O
Properties :
i) It is colourless crystalline solid, soluble in water. Its aqueous solution contains ![]() ions, therefore its aqueous solution gives test of
ions, therefore its aqueous solution gives test of ![]() .
.
ii) Hydrolysis :
Aluminium sulphate of potashalum is hydrolysed to give strong acid H2SO4. Therefore its aqueous solution is acidic. (K2SO4 is not hydrolysed because it is a salt of strong acid and strong base.)
Al2(SO4)3 + 6 H2O ———>2 Al(OH)3 + 3H2SO4
iii) Effect of heat :
It melts at 90 degree, at 200 degree water of crystallisation is given out and a porous substance is formed which is called burnt alum. On redness aluminium sulphate decomposes.
K2SO4 . Al2 (SO4)3 . 24H2O ———> K2SO4 . Al2(SO4)3 + 24H2O
K2SO4 . Al2(SO4)3 ———-> K2SO4 +Al2O3 + 3 SO3
Uses:
i) It is used as mordant in dye.
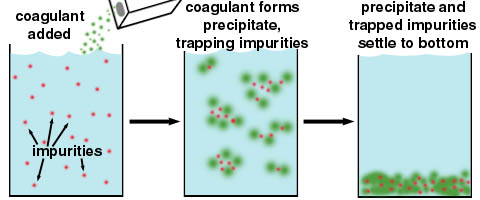
ii) In the purification of water.
iii) In coagulation of blood .
iv) It is also used as an antiseptic agent.
Note: CuSO4 . Fe2(SO4)3 . 24H2O is double sulphate but it is pseudo alum because copper is divalent .
Conversions :
i) Al2O3 to anhydrous Aluminium chloride
Al2O3 + 3C + 3Cl2 ——–> 2AlCl3 + 3CO
ii) Al to anhydrous AlCl3
2Al + 6HCl ——–> 2AlCl3 + 3H2
iii) Potassium sulphate to potash alum
K2SO4 + Al2(SO4)3 + 24H2O ——–> K2SO4 . Al2(SO4)3 . 24H2O
iv) Aluminium chloride to Aluminium bromide :
AlCl3 + 3NH4OH ——–> Al(OH)3 + 3NH4Cl
Al(OH)3 + 3HBr ———> AlBr3 + 3H2O
v) Alumina or Bauxite to PotashAlum :
Al2O3 . 2H2O + 3H2SO4 ——–> Al2(SO4)3 + 5H2O
K2SO4 + Al2(SO4)3 + 24H2O ———> K2SO4 . Al2(SO4)3 . 24H2O
vi) Aluminium to potashalum :
Al + H2SO4 ———> Al2(SO4)3 + SO2 + H2O
K2SO4 + Al2(SO4)3 + 24H2O ———-> K2SO4 . Al2(SO4)3 . 24H2O
vii) Aluminium chloride to aluminium sulphate :
AlCl3 + 3NH4OH ———-> Al(OH)3 +3NH4Cl
2Al(OH)3 +3 H2SO4 ———-> Al2(SO4)3 + 6H2O
viii) Aluminium sulphate to anhydrous AlCl3 :
Al2(SO4)3 + 6NH4OH ———-> 2Al(OH)3 + 3(NH4)2 SO4
![]()
Al2O3 + 3C + 3Cl2 ———-> 2AlCl3 + 3CO
ix) Potash Alum to Al2(SO4)3 :
K2SO4 . Al2(SO4)3 . 24H2O +6 NH4OH ———>2Al(OH)3 +3 (NH4)2SO4 + K2SO4 + 24 H2O
2Al(OH)3 + 3H2SO4 ———-> Al2(SO4)3 + 6H2O
Read more articles at chemistryonline.guru