Arrhenius equation-
source : youtube.com
Arrhenius equation-
According to Arrhenius,
i) All molecules of a system can not take part in a chemical equation .
ii) Only certain number of molecules will react . These reacting molecules are known as active molecules.
iii) The molecules which do not take part in chemical reaction are known as passive molecules.
iv) An equilibrium exists between active and passive molecules.
M (active) ⇌ M (passive)
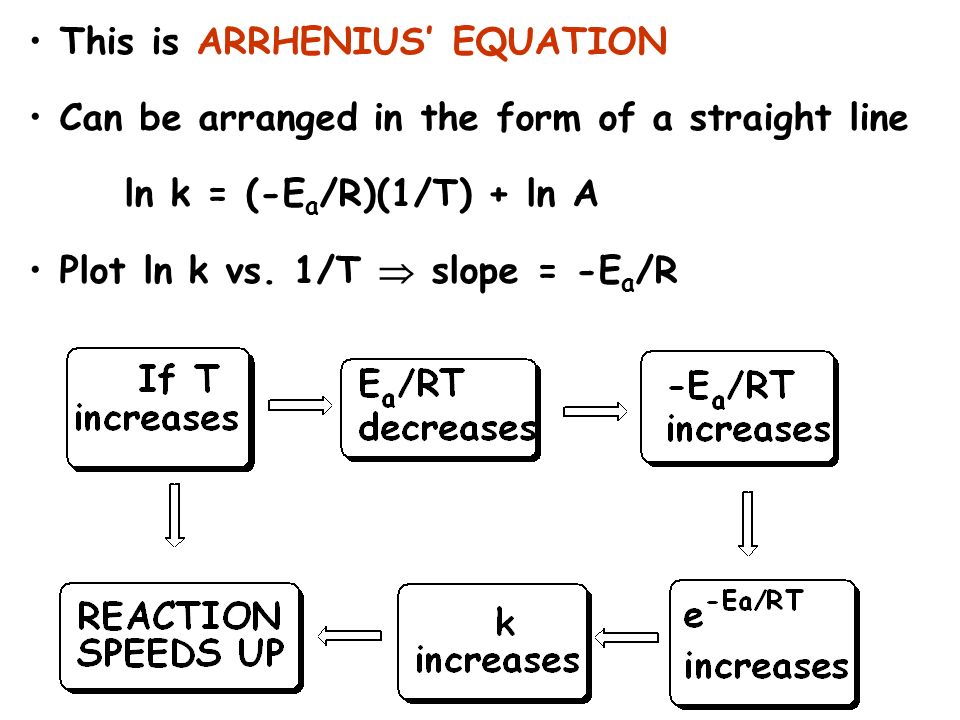
v) When temperature is raised , the above equilibrium shifts to the left then number of active molecules increases. Hence rate of reaction increases with temperature.
So basic concept of Arrhenius theory is that passive molecules becomes active due to absorption of energy. Arrhenius proposed a simple equation known as Arrhenius equation.This equation gives a relationship between rate constant (K) and temperature of the system.
Arrhenius equation is ,
K = Ae -Ea/RT
A is a constant known as frequency factor. It gives frequency of binary collisions of reactant molecules per second per litre.
Ea = Energy of activation , R = gas constant , T = temperature in Kelvin
Another form of Arrhenius equation ,
Taking log of both sides of Arrhenius equation ,
ln K = ln A- Ea/RT
Now convert ln into log,
2.303 log K = 2.303 log A- Ea/RT
log K = (- Ea/ 2.303 RT) + log A
or
log K = log A – (Ea/ 2.303 RT)
As the value of Ea increases, the value of K decreases therefore rate of reaction decreases. As value of T increases, the value of K increases. This equation is used to calculate the value of activation energy .
Calculation of activation energy-
i) Graphical method-
log K = (- Ea/ 2.303 RT) + log A ————eq 1
Eq (1) is a type of ‘ y = mx + c ‘ equation and represents a straight line . If values of ‘log K’ are plotted against ‘1/T’ , a straight line should be obtained . Hence ,
slope = – Ea/ 2.303 R
In order to determine energy of activation of a reaction , its rate constant at different temperatures are measured . Now the values of ‘ log K ‘against ‘1 / T ‘are plotted. The curve obtained is a straight line and slope of line is measured . Slope is related to Ea , as
Slope = -Ea / 2.303 R
By knowing the ‘value of slope’ and ‘R’ , equation.the activation energy can be calculated with the help of above

source : ch302.cm.utexas.edu

source : Chemistry- TutorCircle
ii) Rate constant method-
If K1 and K2 are the rate constants measured at temperatures T1 and T2 respectively , then according to equation,
log K = log A – (Ea/ 2.303 RT) ———–eq 1
log K1 = log A – (Ea/ 2.303 RT1) ————- eq 2
log K2 = log A – (Ea/ 2.303 RT2) ————- eq 3
Subtracting eq( 2) from eq (3)
log K2 – log K1 = (-Ea/ 2.303 RT2) – (- Ea/ 2.303 RT1)
log [K2 / k1] = [Ea/ 2.303 R][(1 / T1) – (1 / T2)] ———- eq 4
By knowing the values of K1 and K2 of a reaction measured at two different temperatures T1 and T2 respectively , the energy of activation (Ea) can be calculated by eq (4).