Inorganic Chemistry
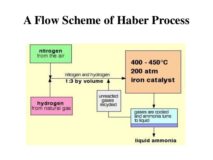
Haber’s Process
Haber’s Process source :chem-guide.blogspot.com Haber’s Process: Principle: NH3 is manufactured by the reaction of N2 and H2. N2 + Continue Reading »

Iodine : Properties
Iodine source : healthworry.com Chemical properties: 1) Reaction with KI : Iodine is soluble in KI due to formation Continue Reading »

Polar and Nonpolar molecules
Polar molecule source : socratic.org Dipole moment: A covalent bond formed between two dissimilar atoms is always polar. A Continue Reading »
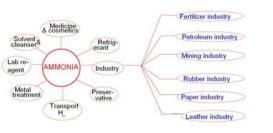
Ammonia : properties
Ammonia source : en.wikipedia.org Chemical properties: 1. Combustion: when mixture of NH3 and O2 burns, it gives N2 gas. Continue Reading »
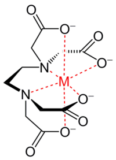
Ligands
Ligands source : chemguide.ca The ions or molecules which surround a metal ion or metal atom are called ligands. They Continue Reading »

Nitrogen, phosphorous, arsenic : Position(P.T)
Nitrogen, phosphorous, arsenic : Position(P.T) source : chemicool.com Nitrogen(N), Phosphorus(P), Arsenic (As), Antimony (Sb) & Bismuth (Bi) are present Continue Reading »

Fajan Rule: Solved examples
Fajan Rule source : microamaze blogspot.com Fajan Rule: Solved examples Q 5: Write decreasing order of Covalent character in LiF Continue Reading »
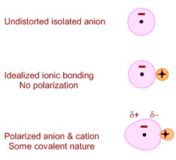
Fajan Rule
Fajan Rule source: chem.uwi.mona.edu.jm Fajan Rule : Covalent character developed in an ionic compound may be determined by fajan’s Continue Reading »

Lead Acetate
Lead Acetate source :scbt Method of Preparation : By the reaction of litharge and acetic acid : Lead acetate Continue Reading »
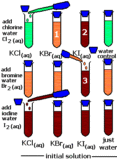
Chlorine : Properties
Chlorine source : slide player.com Chemical Properties of Chlorine : 1) Reaction with dry SO2 : sulphuryl chloride is formed. SO2 Continue Reading »