Fajan Rule
source: chem.uwi.mona.edu.jm
Fajan Rule :
Covalent character developed in an ionic compound may be determined by fajan’s rule. According to this rule covalent character is more , when :
a) Ions have high charge i.e cation has greater positive charge and anion has greater negative charge.
b) Cation has small ionic radius.
c) Anion has large ionic radius.
d) Compounds of cation having s2,p6,d10 configuration of outer most shell are largely covalent as compared to cation having the same size and charge but outer electronic configuration of s2p6 type.
Explanation:
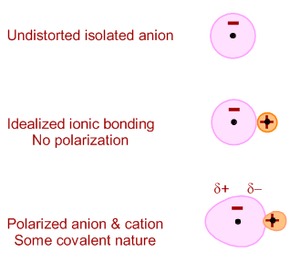
In ionic compound , cation attracts the electrons of anion towards itself. The ability of cation to attract the electrons of anion is called polarizing power. The electrons of anion get attracted towards the cation . This is called polarizability of the anion. Due to this, anion is distorted. It is called polarization. Due to polarization sharing of electrons takes place and ionic compound has some covalent character.
“Greater is the polarization, greater is the covalent character.”
Question 1:Write decreasing order of Covalent character in LiCl, NaCl, KCl.
Solution : LiCl > NaCl > KCl
Reason : According to Fajan Rule ,“Smaller is the cation, more is the covalent character.”
Question 2: Write decreasing order of Covalent character in NaCl , MgCl2 , AlCl3.
Solution : AlCl3 > MgCl2> NaCl
Reason : According to Fajan Rule , ” cation has more positive charge is more covalent.”
Q 3: Write increasing order of melting point of AlCl3 , MgCl2 , NaCl
Solution :
AlCl3 > MgCl2 > NaCl
1800 > 7100 > 8000
Reason : Bigger is the anion, greater is the covalent character. Melting point of covalent compound is less than electrovalent compound.
Q 4: Write decreasing order of Covalent character in AlBr3, AlCl3, AlF3 .
Solution : AlBr3 > AlCl3 > AlF3
Reason : Bigger is the anion, greater is the covalent character.