Chlorine

source : slide player.com
Chemical Properties of Chlorine :
1) Reaction with dry SO2 : sulphuryl chloride is formed.
SO2 +Cl2 ——–> SO2Cl2 (sulphuryl chloride)
2) Reaction with carbon monoxide:
CO +Cl2 ———> COCl2 (carbonyl chloride)
3) Reaction with dry slaked lime Ca(OH)2 : We get bleaching powder
Ca(OH)2 +Cl2 ——>CaOCl2 (bleaching powder) +H2O
4) Reaction with lime water :
a) with cold lime water : calcium chloride & Calcium hypo chlorite is formed.
2Ca(OH)2 +2Cl2 ——–> CaCl2 (calcium chloride) +Ca(OCl2)2 (Calcium hypo chlorite) +2H2O
b) with hot lime water : calcium chloride & Calcium chlorate is formed.
6Ca(OH)2 +6Cl2 ——–> 5CaCl2 +Ca(ClO3)2 ( calcium chlorate)+6H2O
5) Reaction with NaOH :
a) With cold and dilute NaOH : Sodium chloride & sodium hypo chlorite is formed.
2NaOH +Cl2 ——–> NaCl +NaClO (sodium hypo chlorite)+H2O
b) With hot and concentrated NaOH : Sodium chloride & sodium chlorate is formed.
NaOH +Cl2 ————> NaCl + NaClO3 (sodium chlorate) +H2O
6) Reaction with Ammonia :
a) With excess of NH3 : Nitrogen & ammonium chloride is formed .
2NH3 + 3Cl2 ——>N2 +6HCl
6HCl +6NH3 ——–> 6NH4Cl
Equation as a whole-
8NH3 + 3Cl2 ——-> N2 +6NH4Cl
b) With excess of Cl2 : nitrogen tri-chloride is formed which is used as explosive.
NH3 + 3Cl2 ——–> NCl3 (nitrogen tri-chloride )(explosive) +3HCl
Oxidizing Properties:
It is presence of moisture chlorine acts as strong oxidizing agent.
a) It oxidizes H2S into S :
Cl2 +H2O ——–> 2HCl +O
H2S +O ——> H2O +S
Equation as a whole –
Cl2 + H2S ——>2HCl + S
b) It oxidizes ferrous chloride into ferric chloride :
2FeCl2 +Cl2 ——-> 2FeCl3
c) It oxidizes acidic ferrous sulphate to ferric sulphate :
Cl2 +H2O ——->2HCl+ O
2FeSO4 + H2SO4 +O —-> Fe2(SO4)3 + H2O
Equation as a whole –
2FeSO4 +H2SO4 +Cl2 —-> Fe2(SO4)3 +2HCl
d) It oxidizes sodium sulphite to sodium sulphate :
Na2SO3 +Cl2 +H2O ——>Na2SO4 +HCl
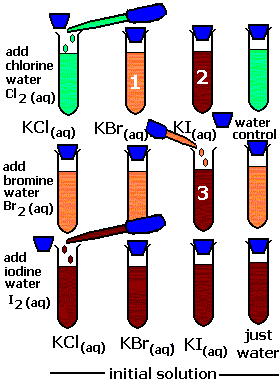
e) It oxidizes KBr into Br2 and KI into I2 :
2KBr +Cl2 ——> 2KCl +Br2
2KI + Cl2 ——> 2KCl +I2
Bleaching Properties:
In presence of moisture Cl2 acts as bleaching agent. Its bleaching action is due to its oxidizing properties. It bleaches colored flowers , leaves , wood pulp etc.
Cl2+ H2O ——–>2HCl + O (Nascent oxygen)
colored substance + O ——-> colorless substance
Test of chlorine:
1. It bleaches colored flowers, leaves, wood pulp etc.
2. It turns starch iodide paper blue or violet. Starch- iodide paper contains starch & KI solution.
2KI +Cl2 ——–> 2KCl + I2.
I2 +starch ——-> blue color.
3. Aqueous solution of chlorine turns blue litmus red.
Uses :
- It is used as oxidizing agent.
- Chlorine acts as disinfectant and germicide.
- As Bleaching agent.