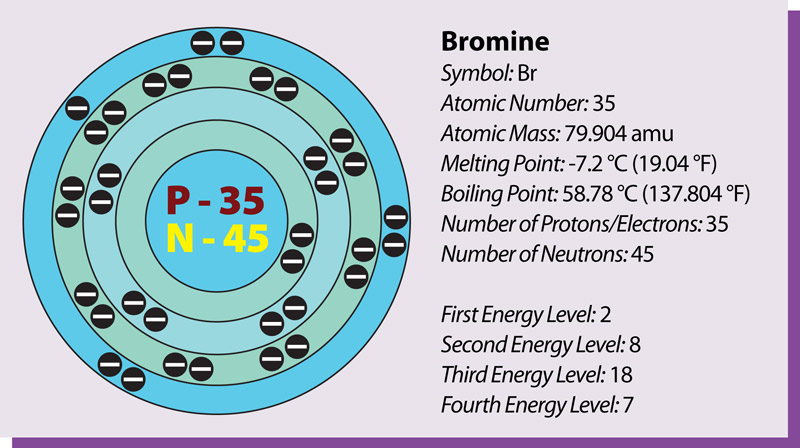
Bromine

source : www.keyword-suggestions.com
Methods of preparation :
1. By passing chlorine gas in aqueous solution of KBr or MgCl2 :
2KBr +Cl2 ———-> 2KCl + Br2
MgBr2 + Cl2 ———> MgCl2 + Br2
2. By the reaction of HCl with a mixture of KBr(potassium bromide) and KBrO3(potassium bromate) :
5KBr + KBrO3 +6HCl ———> 6 KCl + 3Br2 + 3H2O
3. By heating KBr or NaBr, MnO2 with concentrated H2SO4:
2KBr + MnO2 + 3H2SO4 ———> Br2 + 2KHSO4 +MnSO4 +2H2O
or
2NaBr + MnO2 + 3H2SO4 ———> Br2 +2 NaHSO4 +MnSO4 +2H2O
Manufacture from Carnallite:
Carnallite is KCl.MgCl2.6H2O. Carnallite obtained from Stassfurt in Germany also contains MgBr2. Carnallite contains about 0.25% Br2 in the form of MgBr2. On concentrating the aqueous solution of carnallite , crystals of KCl separate out. The filterate contains MgCl2 and MgBr2. This filterate is also called mother liquor.
Hot mother liquor is sprayed from the top of tower which is packed with stone pieces. A mixture of chlorine gas and stream is passed from the bottom of the tower. Bromine is liberated from MgBr2 which is present in the mother liquor.
MgBr2 + Cl2 ———-> MgCl2 +Br2
Vapours of bromine are drawn off from the exist and condensed. Vapours which is not get condensed ,are taken into a chamber containing iron then it reacts with iron and form ferrous bromide and ferric bromide. This mixture is used for the manufacture of KBr.
3Fe + 4Br2 ——-> FeBr2 + 2FeBr3
when mother liquor is sprayed from the top, some liberated bromine dissolves in it and comes in recovery chamber. Bromine collected in recovery chamber is vaporized by steam. The vapours of bromine are passed through the tower again and drawn off from the exist at the top of the tower and condensed.
Manufacture of bromine from sea water :

source : www.octelamlwch.co.uk
Sea water contains KBr , MgBr2 & NaCl. Sea water is concentrated and made acidic and then Cl2 is passed in it.
MgBr2 +Cl2 ———> MgCl2 +Br2
2KBr + Cl2 ——-> 2KCl + Br2
Bromine dissolves in hot water. Hot air is passed in water to vaporize Br2. These vapours are passed in hot aqueous solution of sodium carbonate. A mixture of NaBr & NaBrO3 is obtained in solution.
Na2CO3 + Br2 ——-> NaBr + NaBrO3 +CO2
Solution containing NaBr and NaBr03 is treated with concentrated H2SO4 then bromine is obtained which is condensed and collected.
5NaBr +NaBrO3 +6H2SO4 ——–> 6NaHSO4 + 3Br2 +3 H2O.