Bromine

source : slide player.com
Physical properties of Bromine :
- It is deep red colored, volatile liquid.
- Bromine is poisonous and produces blisters on skin.
- It has pungent odour.
- It is soluble in water. Its aqueous solution is called bromine water.
Chemical properties :
1) Reaction with NH3 : Ammonium bromide & nitrogen is formed.
2NH3 + 3Br2 ——–> N2 + 6HBr
6 HBr + 6NH3 ———–> 6NH4Br
Equation as a whole-
8NH3 +3 Br2 ——-> 6NH4Br +N2
2) Reaction with NaOH :
a)With Cold and dilute NaOH : Sodium bromide & sodium hypo bromite is formed.
2NaOH +Br2 ——-> NaBr +NaBrO (sodium hypo bromite) + H2O.
b) With hot and concentrated NaOH : Sodium bromide & sodium bromate is formed.
6NaOH + Br2 —–>5 NaBr +NaBrO3(sodium bromate) +3 H2O.
3) Reaction with Ba(OH)2 solution (baryta water):
6Ba(OH)2 + 6Br2 —–>5 BaBr2 (barium bromide) +Ba(BrO3)2 (barium bromate)+ 6H2O.
4) Reaction with hot and concentrated Na2CO3 solution : Sodium bromide & sodium bromate is formed.
3Na2CO3+ 3Br2 ——-> 5NaBr + NaBrO3 + 3CO2
5) Reaction with Ca(OH)2 : calcium bromo hypo bromite is formed.
Ca(OH)2 +Br2 ——–> CaOBr2 (calcium bromo hypo bromite) + H2O
6) Oxidizing properties :
In presence of moisture bromine acts as strong oxidizing agent.
a) It oxidizes SO2 into H2SO4:
Br2 +H2O ——->2HBr +O
SO2 + O+ H2O ——–> H2SO4
Equation as a whole-
Br2 + SO2 +2H2O —->H2SO4 +2HBr
b) It oxidizes sodium thio sulphate to sodium tetra thionate:
2Na2S2O3(sodium thio sulphate) + Br2 —-> Na2S4O6( sodium tetra thionate) +2NaBr
c) It oxidizes sodium arsenite to sodium arsenate:
Br2 +H2O ——> 2HBr +O
Na3AsO3 +O ——> Na3AsO4
Equation as a whole-
Br2 + H2O + Na3AsO3(sodium arsenite) ——–> 2HBr +Na3AsO4(sodium arsenate)
d) It displaces I2 from KI:
2KI + Br2 ———>2KBr +I2
Test of bromine:
1) Bromine is red colored liquid with pungent odour.
2) It turns starch iodide paper blue.
2KI +Br2 ——-> 2KBr + I2
I2 +starch ——> blue color
Uses:
1) As oxidizing agent.
2) As a germicide.
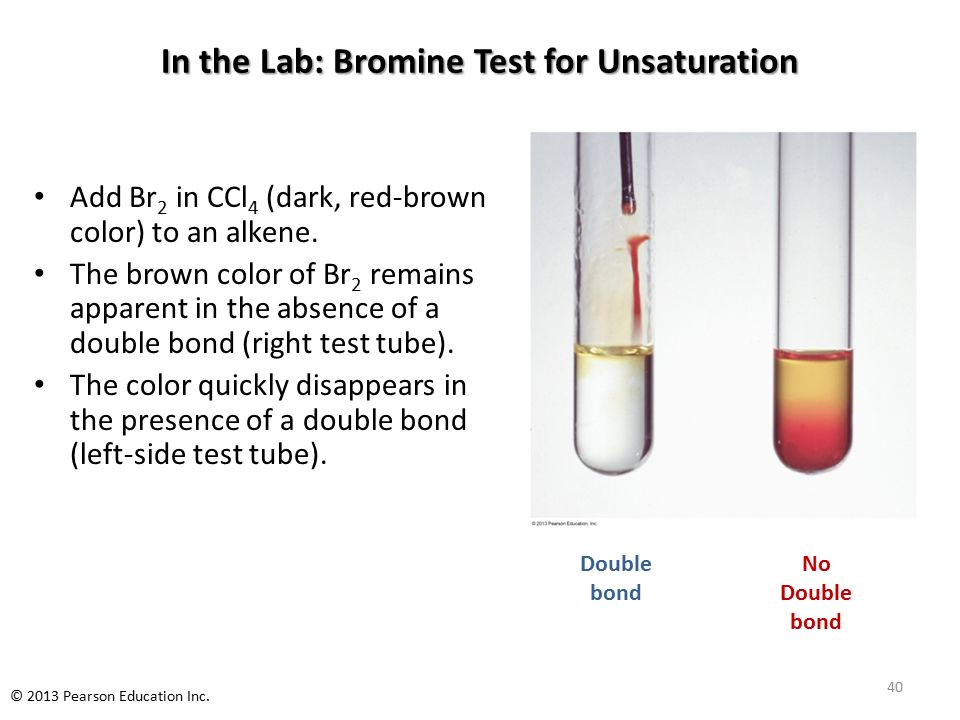
3) Br2-water is used to test unsaturation in organic compounds.