Compound

source : online-sciences.com
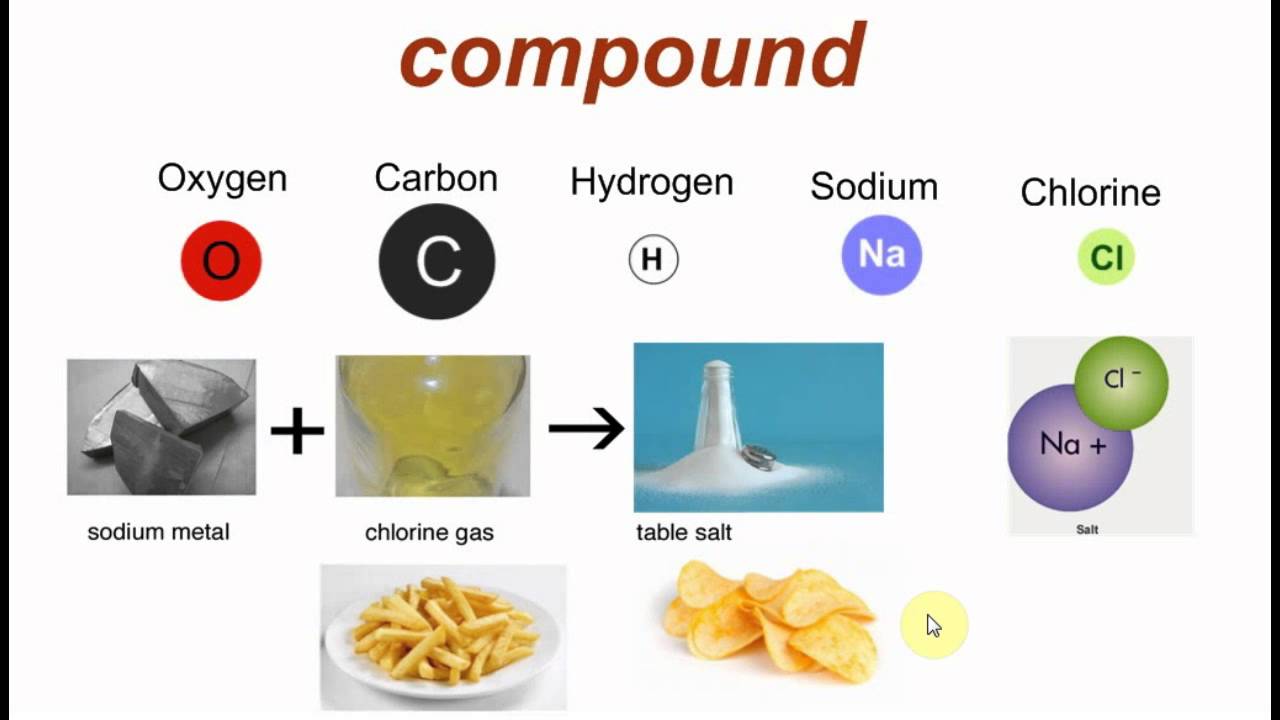
Compound-
Substance made up of two or more elements chemically combined in a fixed proportion by weight.
Ex . Water always contains hydrogen and oxygen in the ratio 1 : 8 by weight.
Carbon dioxide contains carbon and oxygen in the ratio of 12 : 32 or 3 : 8.
Properties of substances-
1) Malleability-
When solid is being beaten then it is converted into sheet .This property of solid is called Malleability.
Ex- copper , silver , gold , aluminium and iron are malleable in nature.
2) Ductility-
It is the property of metal to be drawn into wires.
Ex- copper , silver , gold , aluminium and iron are ductile in nature.
3) Plasticity-
It is the property of solid substances due to which solid does not come back to its original shape, size or volume
after the deforming force is removed.
4) Elasticity-
It is the property of solid substances in which solid regain to its original shape, size or volume after the deforming force is removed.
5) Hygroscopicity-
Compounds combine with moisture of atmosphere and get converted into hydroxides or hydrates. Such compounds are called hygroscopic.
Ex- anhy. CuSO4, anhy. FeSO4, anhy. Na2CO3 , quick lime are hygroscopic in nature.
6) Deliquescence-
Some compounds absorb moisture of atmosphere and become wet. These compounds are called deliquescent.
Ex- Anhy. MgCl2, Anhy. CaCl2, NaOH and KOH are deliquescent in nature.
7) Efflorescence-
Some crystalline solid loses their water of crystallisation on exposure and becomes powdery.Such compounds are called efflorescent.
Ex- Na2CO3.10H2O (washing soda), Na2SO4.10H2O (Glauber’s salt) , K2SO4. Al2(SO4)3. 24 H2O (potash alum) are efflorescent compound.
8) Brittleness-
When solid is converted into small pieces on hammering. Then compound is called brittle.
9) Hardness-
A material is said to be harder than the other if it can scratch it.Hardness is measured in Moh’s scale.For this purpose , ten materials have been selected and assigned hardness from 1 to 10.