Electron Affinity-
source : intro.chem.okstate.edu
Electron Affinity:
“It is equal to the energy liberated when an extra electron is taken by an isolated gaseous atom”. Its unit is eV/atom or KJ/mole or KJ/gm-atom. It is represented by EA or E.
for example:

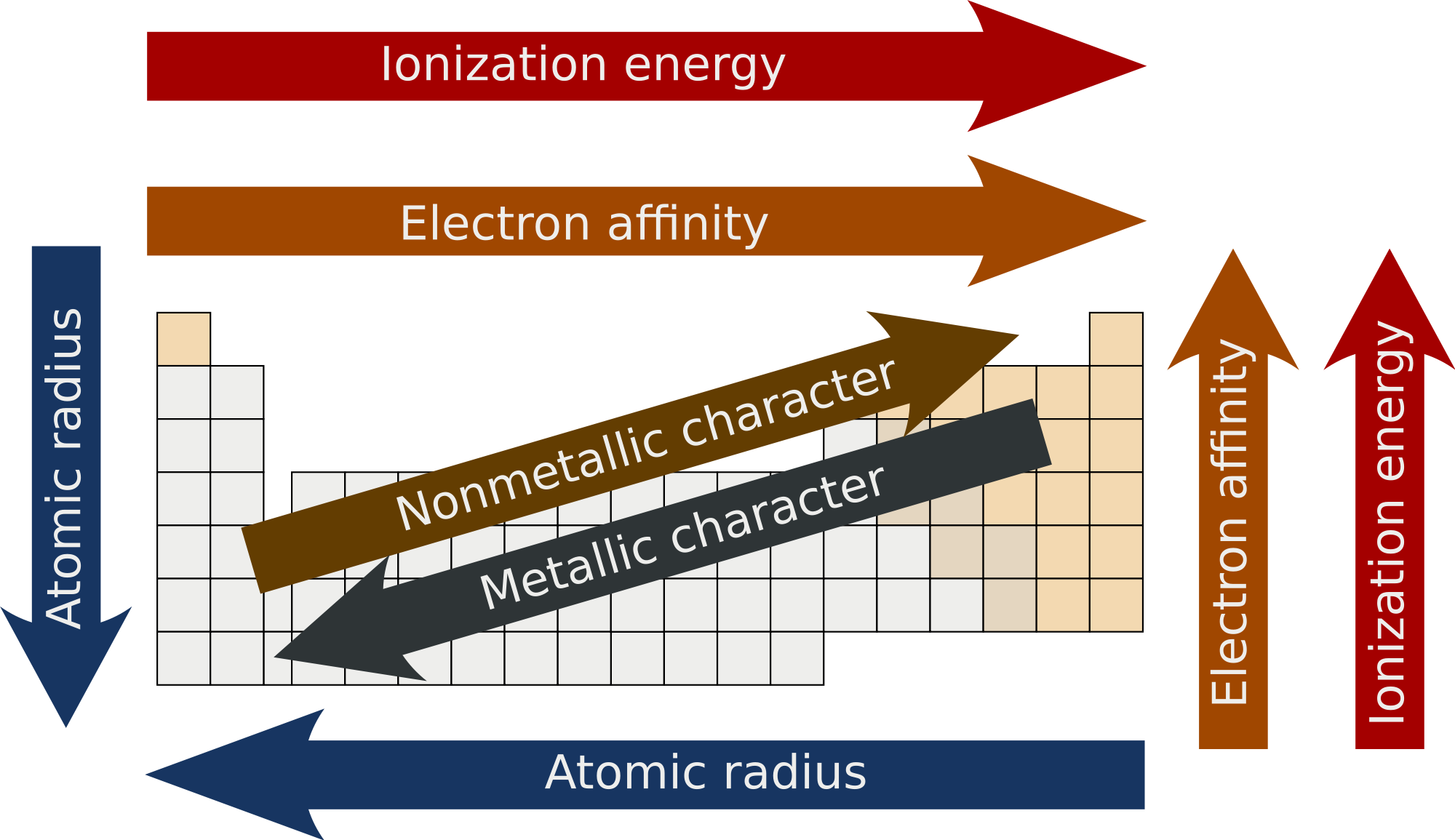
Variation in a group
It decreases on moving down the group because atomic radius of atom increases and tendency to loose electron increases.
variation in a period
It increases in a period on moving from left to right because atomic radius decreases and extra electron added is attracted towards the nucleus by greater force.
Question: Value of second electron affinity is always negative, why?
Solution–Its reason is that energy is to be given to overcome the repulsion between the anion and electron. The negative value of EA shows that energy is to be given to add the electron.
Question:Electron affinity of inert gases is negative. why?
Solution–
Inert gases have stable and closed shell configuration. They do not have tendency to accept electron. If electron is to be gained by inert gases then energy is to be supplied from outside. Hence inert gases have negative value of electron affinity.
Question:Electron affinity of Cl is greater than F. why?
Solution
In a period halogens have highest electron affinity. Electron affinity of Fluorine should be higher than Chlorine but atomic radius of F is small and its electron density is high. Therefore added electron feels repulsion by the electrons of F -atom. This decreases the electron affinity of fluorine.
Note: electron affinity of Cl is highest among all known elements.
Question: Arrange F ,O ,Cl in decreasing order of electron affinity ?
Cl > F > O
Question: Arrange F , O ,N in decreasing order of electron affinity ?
F > O > N
Electronegativity:
Electro negativity is the capacity of an atom to attract the shared electron pair towards itself in a covalent compound .
Variation in a group:
Electronegativity decreases on moving down the group due to increase in atomic radius.
variation in a period:
Electro negativity increases on moving from left to right in a period due to decrease in atomic radius.
Note: halogens have highest electro negativity in a period. F has highest electro negativity among all the known elements.
Question: Arrange F , O , N in the decreasing order of electronegativity ?
F > O > N
Question:
Arrange F , Cl, O in the decreasing order of electronegativity ?
F > O > Cl
Electro negativity and Electron Affinity:
electronegativity deals with atoms in a molecules while electron affinity deals with isolated gaseous atoms.
Read more articles at chemistryonline.guru