Elements – s & p-block

source : wdpunit10tri320125thhr.pbworks
Elements – s & p-block:
Elements of s-block:
Elements of IA and IIA or 1 & 2 group are present in the left side of periodic table are called s-block elements. In these elements last electron enters in outer s-orbital.
Characteristics :
1) Electronic configuration : The electronic configuration of outermost shell is ns1 or ns2.
2) valency : The valency of elements of IA is one and IIA is two .
3) Ionization potential : I.P of s-block element (except H) is low due to large radius because I.P is inversely proportional to atomic radius.
4) Atomic and ionic radius : Radius of s-block elements is large. Radius of cation is always smaller than its neutral atom.
![]()
5) Properties of the cation : Elements of IA and IIA form cations of the type M+ &M2+ respectively.![]() These cations are colorless and diamagnetic because all the electrons present are paired.
These cations are colorless and diamagnetic because all the electrons present are paired.
6) Standard reduction potential : s-block elements have negative standard reduction
potential but standard reduction potential of hydrogen is zero.

7) Reactivity : s-block elements are very reactive. These react with water and acid to liberate H2 gas.
8) Reducing power : I.P and standard reduction potential of s-block elements are small. Hence these elements have tendency to form cation by loss of electrons. Thus s-block elements are powerful reducing agent. Alkali metals ( IA) are more powerful reducing agent than alkaline earth metal (IIA).
9) Nature of oxides and hydroxides : Oxides and hydroxides of alkali and alkaline earth metals are basic but BeO is amphoteric in nature . Example: Li2O , Na2O, CaO, MgO, LiOH , NaOH , Ca(OH)2 , Mg(OH)2
10) Nature of compounds : s-block elements form electrovalent compounds. Beryllium forms covalent compounds.
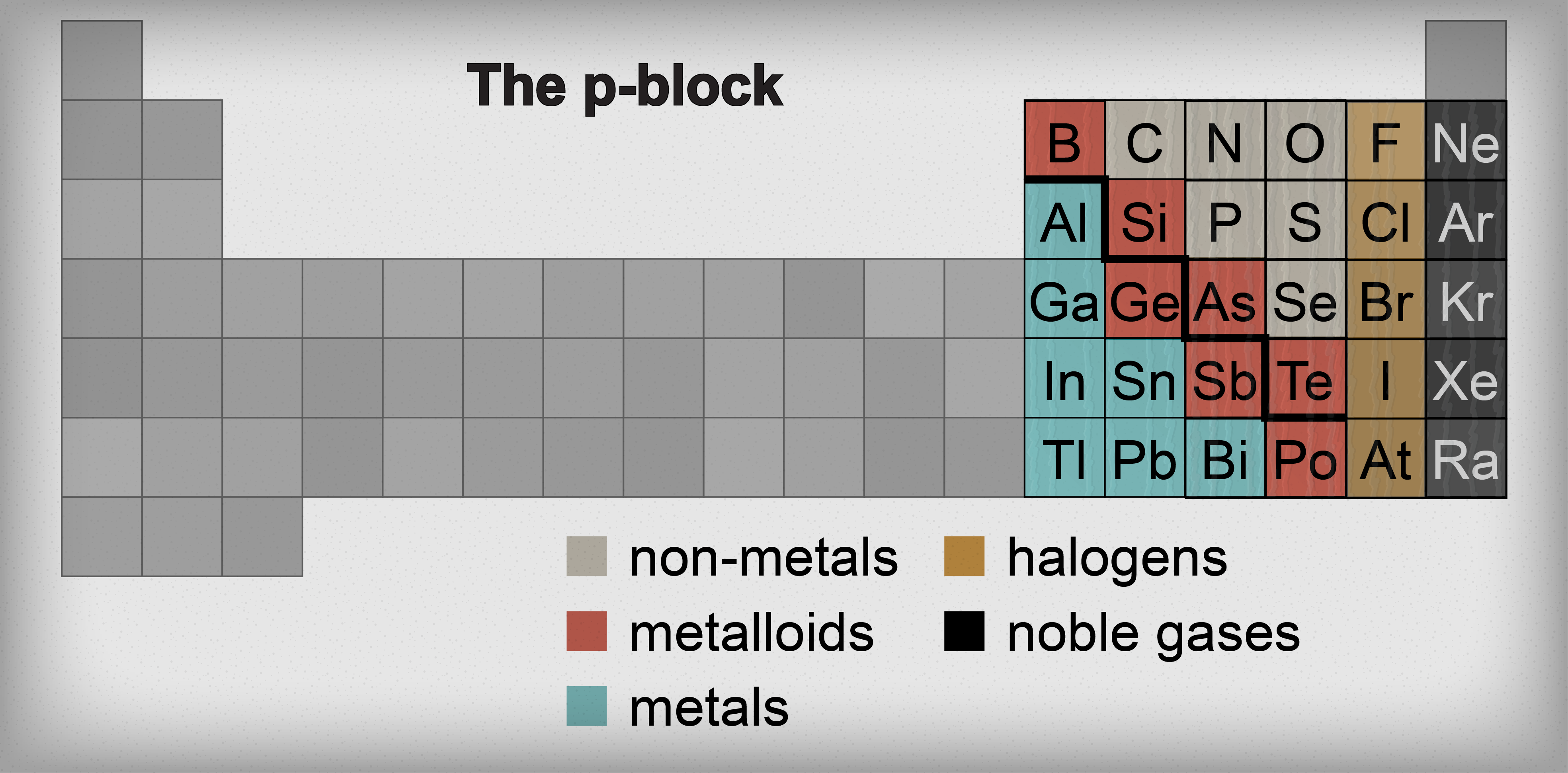
Elements of p-block :
Elements of IIIA, IVA, VA, VIA, VIIA, or elements of groups 13 to 17 are present in the right side of the periodic table. In these elements last electron enters in p-orbital therefore these are p-block elements. Inert gases are also elements of p-block but their properties are entirely different from p-block elements.
Characteristics :
- Electronic configuration of outer most orbit is ns2 np 1-6.

2) Valency : Valency of IIA or 13 group element is 3 ( such as B, Al ). Valency of elements having 4,5,6 and 7 electrons in their outer most shell show valency 4 , 3 , 2 and 1 respectively.
3) Variable valency : Some elements of p-block show variable valency. It is due to inert pair effect or due to presence of vacant d-orbital.
Example: PCl3 & PCl5 , SnCl2 & SnCl4 , SO3 & SO4, Cl2O5 & Cl2O7 etc.
4) Ionization potential : Ionization potential of p-block elements are higher than s-block elements because their atomic radius is smaller than s- block elements & I.P is inversely proportional to atomic radius.
5) Atomic radius : Their atomic radius is smaller than s- block elements.
6) Metallic and Nonmetallic character : Elements of p-block show metallic and nonmetallic properties because they contain metals, nonmetals and metalloids. C , Si (nonmetal) Ge (Metalloids) Sn & Pb (metal).
7) Reactivity : most of the p-block elements are less reactive but some are very reactive such as O,S,P & halogens.
8) Catenation property : The property of forming chain of identical atoms is called catenation property. S8,P4,O3 etc show catenation property . Great catenation property is shown by Carbon.