Empirical Formula
source : slide share.net
Empirical Formula
“The simplest formula of an organic compound which represents its % composition is called its empirical formula or simple formula”.
- An organic Compound contains C= 48%, H= 8% . 0.48 gm. of compd was Kjeldahlised & liberated NH3 requires 19.2 ml N/2 H2SO4. Find the empi. formula
Ans. N =1/2 =0.5
V =19.2 ml
% of N= 1.4 NV/w
=1.4 x 0.5 x 19.2 /0.48
= 28 %
% of O= 100–(48+8+28)
= 16%
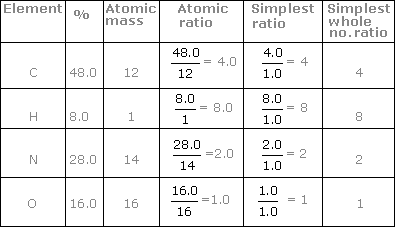
| Element | % | Atomic weight | Relative no. of atoms | Simplest Ratio |
| C | 48 | 12 | 48/12=4 | 4/1=4 |
| H | 8 | 1 | 8/1=8 | 8/1=8 |
| N | 28 | 14 | 28/14=2 | 2/1=2 |
| O | 16 | 16 | 16/16=1 | 1/1=1 |
C:H:N:O=4:8:2:1
Emp. Formula = C4H8N2O Ans.
Q — % of C= 40.68%, H=8.47%, N=23.73% Calculate Empirical Formula ?
Ans. % of 0=100–(40.68+8.47+23.73%) =27.12 %
| Element | % | Atomic weight | Relative no. of atoms | Simplest Ratio |
| C | 40.68 | 12 | 40.68/12=3.39 | 3.39/1.69=2 |
| H | 8.47 | 1 | 8.47/1=8.47 | 8.47/1.69=5 |
| N | 23.73 | 14 | 23.73/14=1.69 | 1.69/1.169=1 |
| O | 27.12 | 16 | 27.12/16=1.69 | 1.69/1.169=1 |
C:H:N:O=2:5:1:1
Emp. Formula = C2H2NO
Q — % of C= 39.13%, H=8.64%, Calculate Empirical Formula?
% of O =100–(39.13+8.64)
=52.23%
| Element | % | Atomic weight | Relative no. of atoms | Simplest Ratio | |
| C | 39.13 | 12 | 39.13/12=3.26 | 3.26/3.26=1 | 1×3=3 |
| H | 8.64 | 1 | 8.64/1=8.64 | 8.64/3.26=2.65 | 2.65×3=8 |
| O | 52.23 | 16 | 52.23/16=3.26 | 3.26/3.26=1 | 1×3=3 |
C:H:O=3:8:3
Emp. Formula = C3H8O3