Enthalpy
source : youtube
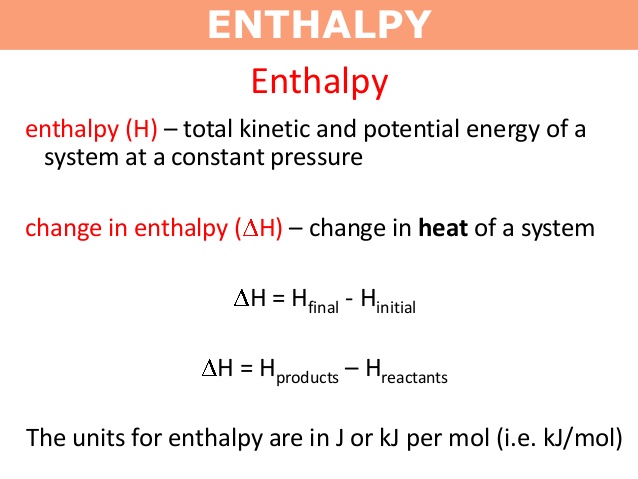
Enthalpy –
To study the heat changes for reactions at constant pressure and at constant temperature, a term enthalpy is used.When a chemical reaction takes place in an open atmosphere,there is change in volume but pressure remains constant.
Enthalpy of a system is defined as,
” The sum of the internal energy and the product of its pressure and volume .”
It is denoted by ‘ H’ and also called heat content.
H = U + PV
U = Internal energy
P=Pressure
V=Volume
Factors affecting Enthalpy-
It depends upon three state functions internal energy,pressure and volume. Enthalpy is also a state function.
A state function is a property of the system , its value depends only upon the state of the system and is independent of yhe path or manner by which the state is reached. It means values of state properties depends upon initial and final states of the system.
Examples- Volume (V),Pressure (P), Temperature (T), Internal energy (U), Enthalpy (H) ,Entropy (S) etc.
Absolute value of enthalpy can not be measured . We can calculate change in enthalpy.Suppose process is
A ———-> B
Then, change in enthalpy,
Δ H = H products – H reactants
Δ H = H p – H r
H p = Enthalpy of products
H r = Enthalpy of reactants
Δ H =Enthalpy change
“The enthalpy change of a reaction is equal to the heat absorbed or evolved during a reaction at constant temperature and pressure.
Δ H is positive if H p > H r and the process or reaction will be endothermic . Δ H is negative if
H r >H p and the reaction will be exothermic.

source: socratic.org
Amount of heat exchanged with the surrounding for a reaction at constant pressure ( i-e Δ H) is different from that exchanged at constant volume ( i-e Δ U) and temperature.
At constant pressure , the volume of the reacting system changes . If volume increases ,the system expands against the atmospheric pressure and energy is required. So a part of energy is used for expansion .The amount of heat exchanged at constant pressure ( i-e Δ H) would be less than amount of heat exchanged at constant volume ( i-e
Δ U).
At constant pressure, work is done on the system and system absorbs some energy from the surroundings.So amount of heat exchanged at constant pressure ( i-e Δ H) is greater than amount of heat exchanged at constant volume ( i-e Δ U).
This relation is very helpful for converting delta H into delta U.
Δ H = Δ U + P ΔV
Δ H = Δ U + Δn g RT