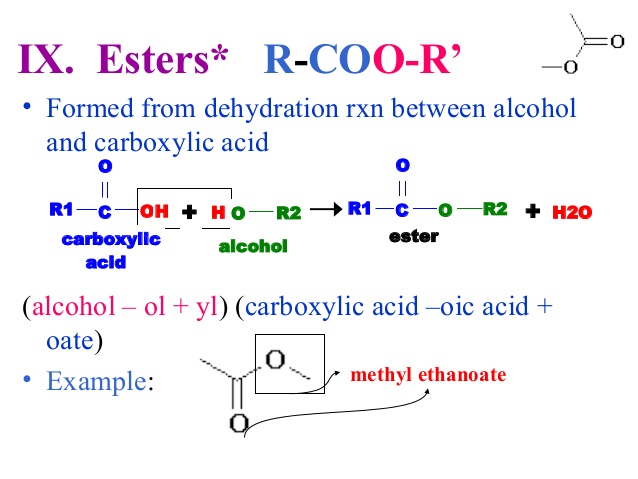
Ester Formation
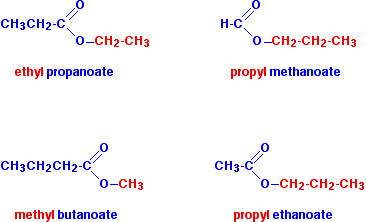
source : chemguide.co.uk
Ester Formation –
The reacn between alc. & acid to form ester is an homogeneous equilibrium in liquid system.
Let ‘a’ moles of Acetic acid &’ b’ moles of ethyl Alcohol ,at equilibrium x moles of ester & x moles of water are formed i.e x moles of CH3COOH & x moles of alcohol have been consumed. Let ‘V’ be the total volume.
CH3COOH(l) + C2H5OH(l)  CH3COOC2H5 (l)+H2O (l)
CH3COOC2H5 (l)+H2O (l)
a/V b/V 0 0
(a-x)/V (b-x)/V x/V x/V
Applying law of mass action,
Kc=[ester][water]/[acid][alcohol]
=[ (x/V) (x/V)]/[c/V)(b-x)/V)]
Kc = x2/(a-x) (b-x)
- In an experiment 1 mole of Acetic Acid & 1 mole of alc. were allowed to react until equilibrium was established. The equilibrium mixture was found to contain 2/3 mole of ester. Calculate the equilibrium constt.
Ans. CH3COOH(l) + C2H5OH(l)  CH3COOC2H5 (l)+H2O (l)
CH3COOC2H5 (l)+H2O (l)
Initial Conc 1 1 0 0
At Equi. 1-2/3 1-2/3 2/3 2/3
=1/3 =1/3
Kc=[ester][water]/[acid][alcohol]
=[ (2/3) (2/3)]/[(1/3)(1/3)]
Kc =4 Ans.
Q.2 At constt. temperature 30 gm. acetic acid & 23 gm. of ethyl alcohol react together. At equilibrium 6gm. H2O is formed Cal. Kc=?
Ans. w of CH3COOH= 30gm
m of CH3COOH=60
w of C2H5OH = 23gm
m of C2H5OH = 46
Initial Concn of CH3COOH = 30/60 mole/l = 1/2 mole/l
Initial Concn of C2H5OH= 23/46 mole/l = 1/2 mole/l
At equilibrium,
Conc. of H2O =6/18 mole/l = 1/3 mole/l
CH3COOH + C2H5OH  CH3COOC2H5 + H2O
CH3COOC2H5 + H2O
Initial Conc. 1/2 1/2 0 0
At. Equi. ( 1/2)-(1/3) ( 1/2)-(1/3) 1/3 1/3
( mole/l) =1/6 =1/6
Kc=[ester][water]/[acid][alcohol]
=[ (1/3) (1/3)]/[(1/6)(1/6)]
Kc =4 Ans.
Q.3 In the reacn
A+B C+D. The initial concentration of A & B are 0.8 mole/l. At equilibrium concentration of C is 0.06 mole/l. Calculate Kc.
Ans. A + B  C + D
C + D
Initial conc. (mole/l) 0.8 0.8 0 0
at Equi. 0.8-0.6 08.-0.6 0.6 0.6
= 0.2 =0.2
Kc =[C][D]/[A][B]
= [0.6 x 0.6 ]/[0.2 x 0.2]
=9 Ans.
- In an experiment at 1000C 1 mole of acetic acid, 1 mole of ethyl A/c & 1 mole of H2O are taken. At equilibrium 54.3 % acid is converted into ester. Calculate the Kc=?
Ans. Initial moles of acetic acid=1 mole
Initial moles of C2H5OH=1 mole
Initial moles of H2O = 1mole
At equilibrium,
moles of Acid converted to ester=( 1 x 54.3) /100 =0.543 mole
CH3COOH + C2H5OH  CH3COOC2H5 + H2O
CH3COOC2H5 + H2O
Initial Conc. 1 1 0 1
At. Equi. ( 1-0.543)/V ( 1-0.543)/V 0.543/V ( 1 +0.543)
=1.543/V
Kc=[ester][water]/[acid][alcohol]
= [ (0.543 ) (1.543)] / [(0.457 )(0.457)]
Kc = 4.0 Ans.