STABILITY – ORBITALS

source : learn next.com
STABILITY – ORBITALS
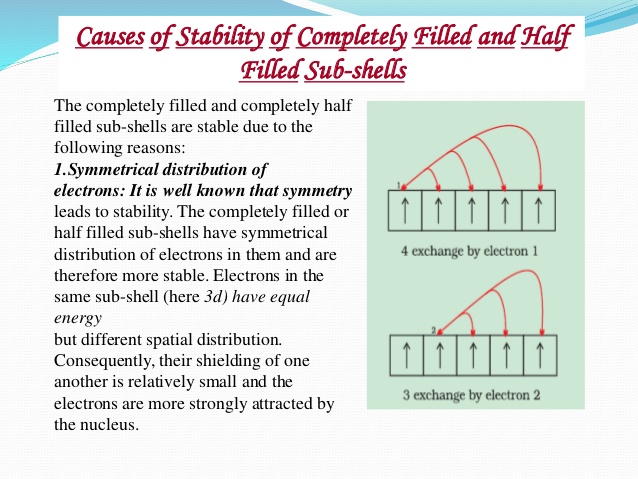
The stability of half filled orbitals ( p3, d5, f7 ) and full filled orbitals ( p6, d10, f14 ) depends upon the symmetrical distribution of charge, symmetrical distribution of charge decreases the energy of the system & hence its stability is increased. To understand the effect of the symmetrical distribution of charge in orbitals, we consider the shapes of orbitals.
The s-orbital is spherical in shape & hence the electronic charge would be distributed uniformly in all directions.
In the three p-orbitals, px, py & pz are symmetrical along. x , y & z axis respectively. In px’, the electronic charge would be concentrated along x-axis, Similarly in py & pz configuration, the electronic charge would be mainly concentrated along ‘y’ & ‘z’ axis respectively. In px2 py’ pz’ configuration the electronic charge would be more concentrated along x-axis. In px2 , py2, pz1 configuration, the electronic change would be more in plane xy . So in all these p-configurations, the distribution of charge is unsymmetrical.
The distribution of charge in px1 py1 pz1 & px2 py2 pz2 configuration would be symmetrical in all directions. So symmetrical configuration of change decreases the energy & hence higher stability.
Similarly the configuration of d-obitals d5 & d10 have symmetrical distribution of charge.
half filled orbitals Full filled d-orgitals
So these configuration would be more stable than other d-configurations.
Therefore half filled & full filled orbitals are more stable than other electronic configuration.
For Ex.
- 24Cr– 1s2, 2s2, 3s2, 3p6, 4s2, 3d4 (expected)
24Cr – 1s2, 2s2, 2p6, 3s2, 3p6, 4s1, 3d5 (Correct)
29Cu– 1s2, 2s2, 2p6, 3s2, 3p6, 4s1, 3d9 (expected )
29Cu– 1s2, 2s2, 2p6, 3s2, 3p6, 4s1, 3d10 (correct)
Half filled & full filled orbitals have lower energy & hence higher stability, therefore , to acquire extra stability one electron from 4s orbital goes into 3d orbital which become half filled or full filled.