Estimation of Elements (Quantitative Analysis)

source : tutor vista.com
Estimation of C&H
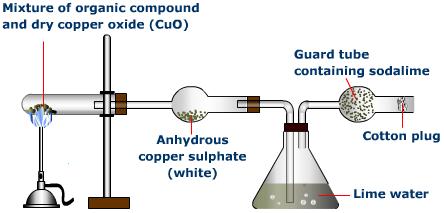
Carbon & Hydrogen are estimated simultaneously by Liebig’s combustion method
Principle
When a known weight of an organic compound is strongly heated with cupric oxide. C& H present are oxidised to CO2 & H2O respectively. Lime water or KOH is used to absorb obtained CO2 while Anhy. CaCl2 or Anhy. CuSO4 is used to absorb H2O. Increase in the wt. of lime water or KOH is the wt. of CO2 while increase in the wt. of Anhy. CaCl2 or Anhy. CuSO4 is the weight of H2O.
CxHy +O2 —–> xCO2 +y/2[H2O]
Calculation of % of C
Let the mass of 0rganic compound is w gm.
Increase in the mass of KOH = Amount of CO2=x gm
44 gms CO2 contain C = 12 gm
x gm CO2 contain C =12x /44 gm
12x /44 gm of carbon is obtained from w gm of o.c.
w gm. 0rganic compound contain C = 12x /44 gm
100gm o.c. contain C = ( 12x /44 w) . 100
% of c=( 12 /44 )[( wt. of CO2) /( wt. of o.c.)100
Calculation of % of Hydrogen
Let the mass of o.c. is w gm.
Increase in the mass of Anhy. CaCl2 or Anhy . CuSO4 = wt of H2O= y gm.
18 gm of water contain H= 2 gm
y gm of water contain H = 2y/18 gm. =
2y/18 gm of H are present in w gm of o.c
w gm o.c contain H= 2y/18 gm
100 gm o.c contain H = ( 2y /18w) . 100 =
% of H= ( 2 /18 )[( wt. of H2O) /( wt. of o.c.)]100
% of O =100 – (sum of % of all elements present in o.c)
Q 1) 0.2475 gm of an o.c on combustion gave 0.4950 gm of CO2 & 0.2025 gm of H2O. Determine the % composition of compound.
Ans .
wt.of.CO2=0.4950 gm.
wt.of H2O= 0.2025 gm.
wt.of o.c. =0.2475
% of C=( 12 /44 )[( wt. of CO2) /( wt. of o.c.)100
=( 12 /44 )[(.4950) /(.2475)]100
=54.54%
% of H =( 2 /18 )[( wt. of H2O) /( wt. of o.c.)]100
percentage of H =( 2 /18 )[( .2025) /(.2475)]
% of H = 9.09%
% of O=100 – (54.54+9.09)=36.41%
Q 2) 0.39gm. hydrocarabon on combustion gave 1.32 gm of CO2 & 0.27 gm of H2O. Calculate percentage composition of hydrocarbon?
Solution:
weight of CO2 =1.32 gm.
wt. of H2O =0.27 gm.
wt. of o.c. =0.39 gm.
% of C=( 12 /44 )[( wt. of CO2) /( wt. of o.c.)100
=( 12 /44 )[(1.32) /(.39)]100
=92.3%
% of H =( 2 /18 )[( wt. of H2O) /( wt. of o.c.)]100
=( 2 /18 )[( 0.27) /(.39)] 100
% of H = 7.7%
% of C =92.3, % of H=7.7%
Q 3) 0.46 gm. of an 0.c containing C, H & O was analysed by combustion method. The increase in mass of Anhy. CaCl2 & KOH are 0.54gm & 0.88gm respectively. Determine % composition of the compound ?
Ans.
weight of CO2 =0.88 gm.
wt.of H2O=0.54 gm.
weight of o.c.=0.46
% of C=( 12 /44 )[( wt. of CO2) /( wt. of o.c.)]100
=( 12 /44 )[(0.88) /(0.46)]100
=52.17%
% of H =( 2 /18 )[( wt. of H2O) /( wt. of o.c.)]100
percentage of H =( 2 /18 )[( 0.54) /(0.46)] 100
% of H = 13.04%
% of O=100 – (52.17+13.04)=34.79%
% of C=52.17, % of H= 13.04, % of O=34.79%
Read more articles at chemistryonline.guru