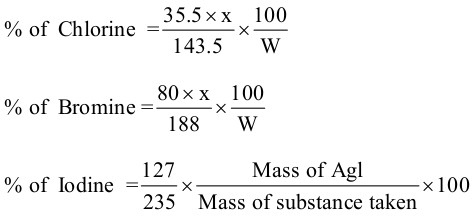Estimation of halogen

source : slide share.net
Estimation of halogen
Estimation of hologen is done by following two methods
(1) Carius Method (2) Piria & Schiff’s method
1) Carius Method
In this method organic compound containing halogen is heated in a carius tube with F HNO3 in presence of AgNO3, Silver halide (AgX) is precipitated. By Knowing the wt. of silver halide & organic Compound percentage of halogens can be calculated (x=-Cl, -Br,-I)
Calculation –
wt. of organic compound (o.c.) = w gm
wt. of AgCl= x gm
Mol. wt. of AgCl=108+35.5 = 143.5
143.5 gm of AgCl contain Cl = 35.5 gm
x gm of AgCl contain Cl =35.5 x /143.5
w gm of o. c .contains Cl = 35.5 x /143.5
100 gm of o. c .contains Cl =[( 35.5 x) 100 ] / (143.5 w)
% of Cl = (35.5 x wt.of AgCl x 100) /( 143.5 x wt. of o.c.)
General Formula
% of X= (At.wt. of X x wt.of AgX x 100) /( mol.wt. of AgX x wt. of o.c.)
At. wt of Br= 80
Molecular weight of AgBr = 108+80= 188
% of Br =(80 x wt.of AgBr x 100) /( 188 x wt. of o.c.)
Atomic weight of Iodine = 127
Molecular weight of AgI = 108+127=235
% of I= (127 x wt.of AgI x 100) /( 235 x wt. of o.c.)
-
Piria & Schiff’s Method-
Known amount of organic compound is fused with CaO+Na2CO3 in Pt or Ni crucible. The product obtained is dissolved in excess of HNO3 & halogen is precipitated with AgNO3 Soln in the form of AgX. By knowing the wt. of o.c . & AgX, We can calculate the % of halogen.
Q 1) 0.185 gm of organic compound gave 0.319 gm AgBr. Calculate the % of Br?
Solution : wt.of AgBr =0.319 gm.
weight of o.c. =0.185 gm.
% of Bromine =(80 x wt.of AgBr x 100) /( 188 x wt. of o.c.)
percentage of Br = (80 x 0.319 x 100) /( 188 x 0.185)
% of Br =73.36 % Ans.
Q 2) 0.156 gm of o. c. on heating with f HNO3 & AgNO3 gives 0.235 gm of AgI. Calculate % of I.
Solution : wt. of AgI=0.235 gm.
weight of o.c.=0.156 gm.
% of Iodine= (127 x wt.of AgI x 100) /( 235 x wt. of o.c.)
percentage of I= (127 x 0.235 x 100) /( 235 x 0.156)
% of I = 81.41 % Ans.
Q 3) 0.1170 gm of an o.c on heating with conc. HNO3 & AgNO3 in carius tube gave 0.42 gm of AgCl. Find the percentage of Cl ?
Ans. wt.of AgCl =0.42 gm.
wt. of o.c.= 0.1170 gm.
percentage of Cl = (35.5 x wt.of AgCl x 100) /( 143.5 x wt. of o.c.)
= (35.5 x 0.42 x 100) /( 143.5 x 0.1170)
% of Chlorine = 88.80 % Ans.
Q 4) 1.09 gm of an organic compound on combustion gave 0.88 gm of CO2 & 0.45 gms. of H2O . 0.218 gm of this compound on heating with f HNO3 & AgNO3 gave 0.376 gm of AgBr. Calculate the % of C, H & Br.
Ans. wt. of CO2 =0.88 gm.
weight of o.c. =1.09 gm.
% of Carbon =(12 x wt.of CO2 x 100) /( 44 x wt. of o.c.)
percentage of C =(12 x 0.88 x 100) /( 44 x 1.09)
% of C =22.01 % Ans.
percentage of H =(2 x wt.of H2O x 100) /( 18 x wt. of o.c.)
=(2 x 0.45 x 100) /( 18 x 1.09)
% of H =4.58 % Ans.
percentage of Br =(80 x wt.of AgBr x 100) /( 188 x wt. of o.c.)
=(80 x 0.376 x 100) /( 188 x 0.218)
% of Bromine=73.39 Ans.