Aluminium

source : docstoc.com
Aluminium : Metallurgy
1] Minerals and ore :
Bauxite: Al2O3 . 2H2O
Diaspore: Al2O3 . H2O
Corundum: Al2O3
Cryolite: Na3AlF6
Alunite: K2So4 . Al2(So4)3 . 4Al (OH)3
Flespar: K2O . Al2O3 . 6SiO2 or KAl Si3O8
2] Extraction:
Main ore of Aluminium is Bauxite. The process involves three steps:
a) Preparation of pure alumina from bauxite
b) Electrolysis of alumina
c) Purification of aluminium
A) Preparation of pure alumina from bauxite :
Bauxite is purified by following methods:
i) Baeyer’s method :
When ferric oxide (Fe2O3) is present as impurity in bauxite then Bayer’s process is used . Bauxite is ground into fine powder and roasted (heating of ore in presence of air) , then ( if present) FeO present is oxidised to Fe2O3
Roasted ore is heated with NaOH solution at 150 degree C then sodium meta aluminate is formed . Impurity of Fe2O3 remains behind. if small amount of silica is present then it reacts with NaOH to form soluble sodium silicate.
FeO ——-> Fe2O3
Al2O3. 2H2O +2 NaOH ———> 2NaAlO2 + 3 H2O
2NaOH + SiO2 ———-> Na2SiO3 + H2O
Impurities are filtered off. The filtrate contains sodium meta aluminate and Na2SiO3. Small amount of freshly prepared aluminium hydroxide is added to the filtrate then sodium meta aluminate is hydrolysed and white precipitate of aluminium hydroxide are obtained. Sodium silicate remains in the solution.
NaAlO2 +3 H2O ———-> Al(OH)3(aluminium hydroxide) + NaOH
Precipitate of Al(OH)3 is filtered, washed, dried and heated at 1200 degree – 1500 degree C then pure alumina is obtained.
Al(OH)3 ———> Al2O3 + H2O
ii) Serpak process :
This process is used when mainly impurity of silica is present in bauxite. Bauxite is mixed with carbon and heated in presence of nitrogen at 1800 degree. Impurity of silica is removed in the form of volatile silicon and aluminium nitride is formed.
Al2O3 . 2H2O + 3C + N2 ——-> 3CO + 2AlN + 2H2O
SiO2 + 2C ——–> 2CO + Si (silicon)
Aluminium nitride is hydrolysed by hot water then precipitate of Al(OH)3 is obtained which is filtered, washed, dried and heated to 1200 degree to 1500 degree then pure aluminium is obtained.
AlN + 3H2O ——–> Al(OH)3 (white ppt.) + NH3
2Al(OH)3 ———> Al2O3 + 3H2O
iii) Hall’s process :
This process is used when both Fe2O3 and SiO2 is present as impurity. Bauxite is fused with Na2CO3 then sodium aluminate is obtained. The molten mass is dissolved in boiling water. Impurity of Fe2O3 is filtered out. the filtrate contains sodium meta aluminate and Na2SiO3
Al2O3 . 2H2O + Na2CO3———>2 NaAlO2 + CO2 +2 H2O
Na2CO3 +SiO2 ——> Na2 SiO3 + CO2
CO2 is passed in the solution at 50-60 degree then precipitates of Aluminium hydroxide are obtained. Precipitates are filtered and heated at 1200 degree to 1500 degree to get pure Al2O3
2NaAlO2 + 3H2O + CO2 ——-> 2Al(OH)3 + Na2CO3
2Al(OH)3 ——–> Al2O3 + 3H2O
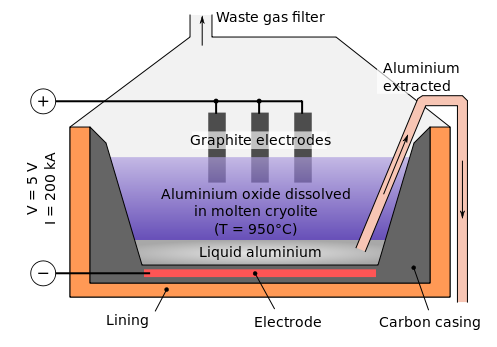
B) Electrolysis of Alumina :
Melting point of pure Al2O3 is very high i.e 2050 degree. Therefore cryolite (Na3AlF6) and fluorspar (CaF2) is added in Alumina to lower its melting point upto 875 – 925 degree . Cryolite also increases the conductance.
Electrolysis of Al2O3 is carried in an iron box. It is lined with carbon which acts as cathode. Graphite acts as anode. These are dipped in electrolyte. A mixture of Al2O3, cryolite and fluorspar acts as electrolyte. A controlling lamp is placed parallel to the cell. When the concentration of Al2O3 decreases, the controlling lamp lights up.

On passing current, Al is deposited at cathode and O2 is liberated at anode. Al is collected from the bottom of the iron box. O2 liberated at anode reacts with graphite rod and forms CO2 and CO & light . Thus carbon of graphite anode is consumed . Therefore graphite rod needs replacement after sometime. To remove the problem of light, coke powder is added in electrolyte.
C) Purification of Aluminium :
Purification of Aluminium is carried out by Hoope’s process.
Hoope’s process :

source : cikgu wong.blogspot.com
Cell contains an iron box which is lined with carbon. The cell contains three layers. The bottom layer contains impure aluminium which acts as anode. The middle layer contains mixture of molten, Sodium fluoride ( NaF), Barium fluoride ( BaF2) & Aluminium fluoride ( AlF3) . This mixture acts as electrolyte. The upper layer contains pure Al2O3 which acts as cathode. The specific gravity of upper layer is lowest while that of bottom layer is highest. On passing current Al3+ from middle layer are reduced at cathode and equivalent amount of Al from bottom layer comes in the middle layer. Thus pure Al from bottom layer deposits at the upper layer & impurity remains behind. Aluminium obtained is 99.98% pure.