Gas Equation

source :theyoungscottishphysicist.co.uk
According to Boyle’s law
V ∝ 1/P (T is constant)
According to Charle’s Law
V ∝ T (P is constant)
Combining these two laws,
V ∝ T/P
PV ∝ T
PV = KT
PV/T = constant
K is a constant
For one mole of gas, the constant K is replaced by R. Since the value of R is same for each gas, it is known as universal gas constant
PV = RT ———gas eq.
P=Pressure of gas
V= Vol. of 1 mole of gas
T= Temperature in Kelvin (Absolute Temp.)
R= Universal gas constant
Gas Eq. for n mole of gas,
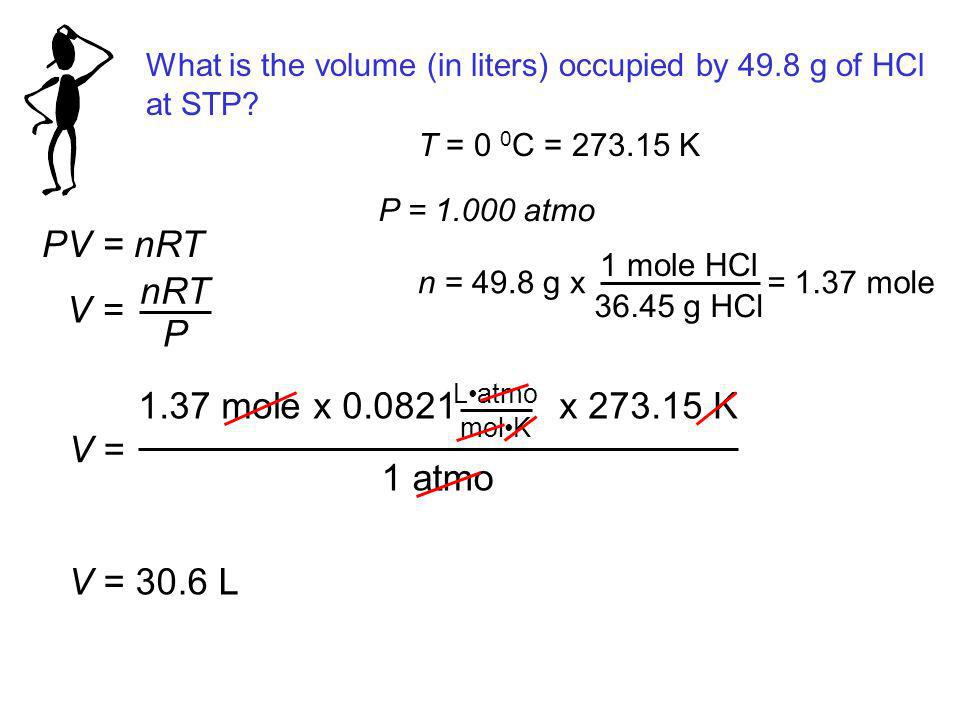
PV =nRT ( Gas equ. )
n= no. of moles of Gas
Above gas eq. may be written as,
n = w/m
PV = w/m (RT) ——–gas equation
w=weight or mass in grams
m=molecular weight of gas
density of gas d = w/v
P = DRT/m gas equ.