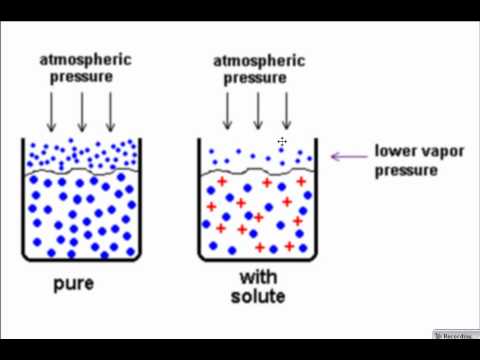
Raoult’s law
source : chem wiki.uc davis.edu
Raoult’s law – Numerical
Question1) The vapour pressure of water at 200C is 17.54 mm. When 20 gm. of a nonvolatile solute is dissolved in 100 gm. of water, the vapour pressure is lowered by 0.30 mm what is the molecular weight of substance?
Solution:
p=17.54 mm.
p-ps =0.30
w = 20 gm
M of H2O = 2+16=18
m = ?
W= 100gm.
(p-ps) /p =wM/Wm
0.30/17.54 =20 x18 /100m
m =210.48 Ans.
Question2 ) The vapour pressure of pure Benzene at certain temp. is 640 mm Hg. A non volatile non electrolyte solid weighing 2.175 gm. is added to 39.0 gm. of Benzene. The vapour pressure of solution is 600 mm Hg. What is the molecular weight of solid substance?
Solution:
p = 640 mm
w= 2.175 gm
W= 39.0 gm
ps=600 mm
m=?
M of benzene = 12×6+1×6 = 72+6 = 78
(p-ps) /p =wM/Wm
( 640-600)/640 = (2.175 x 78 ) / 39 m
m= 69.6 Ans.
Question3) The vapour pressure of dilute aqueous solution of glucose is 750 mm of Hg at 373K. Calculate mole fraction of solute?
(Vapour pressure of pure water at 373 K= 760mm.)
Solution:
p =760 mm
ps =750 mm
( p-ps) / p = mole fraction of solute (X1)
760-750/750 =X1
X1 = 10/750
X1= 0.013 Ans.
Read more articles at chemistryonline.guru