orbital
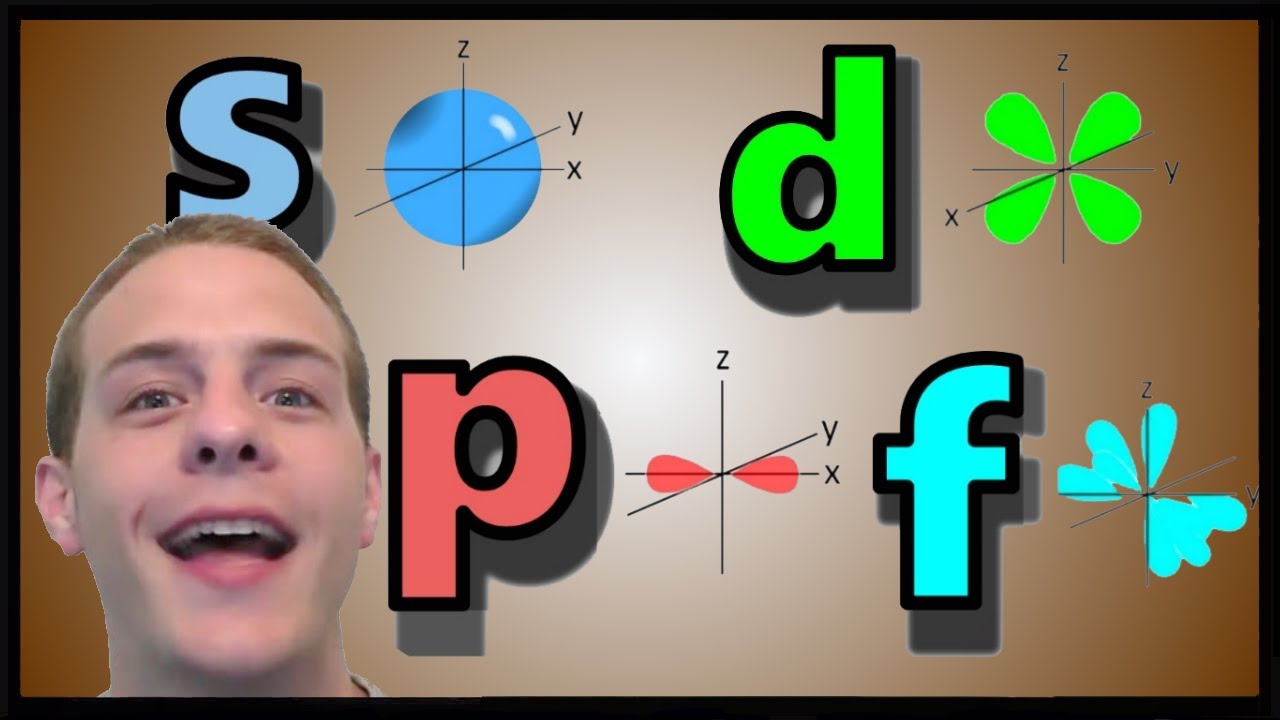
source : youtube.com
Orbit
A circular path at a definite distance from the nucleus in which electron revolves around the nucleus is called orbit.
n=1,2,3,4,….., etc.
Orbital
The region in three dimensional space around the nucleus where the probability of finding electron is maximum, is called orbital.
Shape of Atomic Orbitals
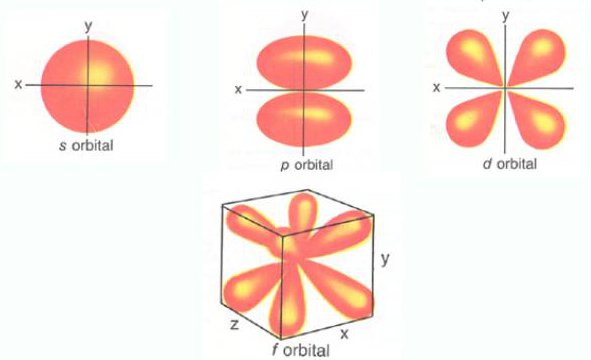
source : www.dirk bertel.com
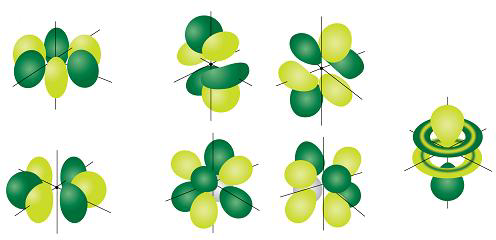
s-Orbital
It is spherical is shape, in which probability of finding electron is identical in all directions from the nucleus. It has only one orientation . s-orbitals are spherically symmetrical so electron density is not concentrated along any axis. All s-orbitals are spherical (similar) shape but size increases with increase in value of Principal Quantum No.

source : en.wiki books.com
p-Orbitals :
The p- orbital is dumb-bell in shape, it consists of two lobes, one on each side of the nucleus. The probability of finding an electron is equal in both the lobes. The p-orbitals have been denoted as px , py & pz depending upon the orientation.
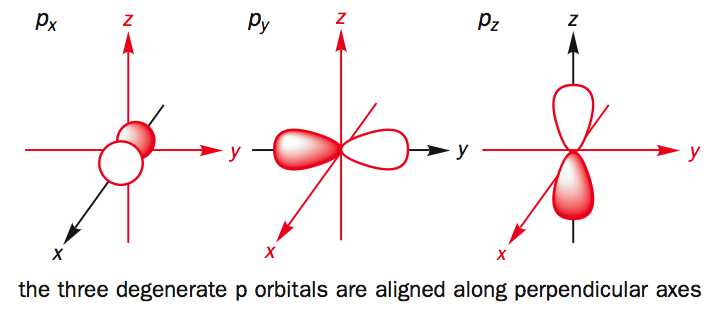
source : liver pool.ac.uk
d-orbitals
The five d-orbitals are equivalent in energy , its shape is like double dumb-bell. The d-orbitals are denoted as dxy, dyz, dzx, dx2-y2 & dz2 , in presence of magnetic field d-orbitals split into two groups such as dxy, dyz, dzx, or t2g & dx2-y2 & dz2 or eg
source: chemed.chem.purdue.edu
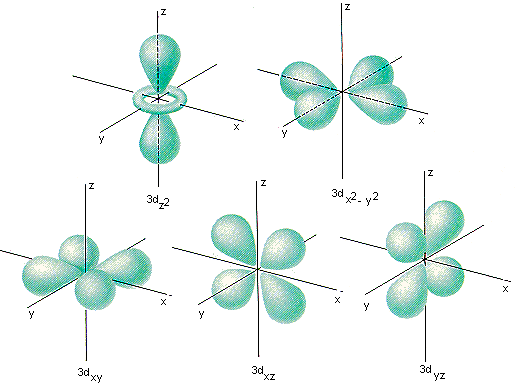
f-orbitals-
The seven f-orbitals are equivalent in energy. These orbitals are denoted as
| fz3 | fxz2 | fyz2 | fxyz | fz(x2−y2) | fx(x2−3y2) | fy(3x2−y2 |
|---|
Their shape is very complicated.
en.wikibooks.org
Read more articles at chemistryonline.guru







