Group displacement law

source : sciencehq.com
pharmalife.weebly.com
Soddy, Fajan & Russel gave a law, to know the position of new element formed after the emission of alpha & beta particle. According to Group displacement law.
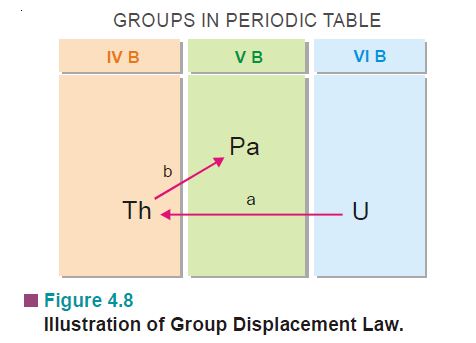
- If an α-particle is emitted by a radio active element from its nucleus, the atomic no. of new element or daughter element formed is decreased by 2 units & atomic weight is decreased by 4 units. Therefore the position of new element formed is displaced by two groups towards the left in the periodic table
ZAM——>Z-2CM-4 +2He4 (α-particle)
- If a β–particle is emitted by a radioactive element, the atomic number of daughter element or new element is increased by one unit. Therefore the position of new element is displaced by one group towards the right in periodic table.
ZAM——> Z+1CM + -1β0 (β-particle)
- If an α-particle is emitted from the nucleus of radioactive element and then 2β-particles are emitted in next two transformations, the daughter element is an isotope of parent element. The daughter & parent element have the same atomic number. Hence according to Group displacement law position of daughter & parent element in the periodic table will remain same.
ZAM—–> ZAM-4 +2-1β0+2He4
- Group displacement law is not applicable to lanthanides & actinides. (f-block elements)
Question 1 ) 84Po215 emits an α–particle from its nucleus. Find the position of new element in the periodic table. Polonium is present in 6th group of periodic table.
Solution )
When α-particle is emitted, the atomic No. of new element is decreased by 2 units & atomic weight by 4 units. Its position is displaced by two group towards left in periodic table
84Po215 ——-> 82Pb211+ 2He4(α-particle)
(VI Group) (IV group)
New element 82Pb211 is present in IV group of periodic table.
- 83x209—alpha—-> Y – -beta–> Z
(V group) (III group) (IV group)
Question 2 ) What is the atomic number & mass no. of daughter elements. Find their position in periodic table X is present in Vth group of periodic table.
Solution )
83x209 — alpha—–>81Y205 —beta——> 82Z205
(V group) (III group) (IV group)