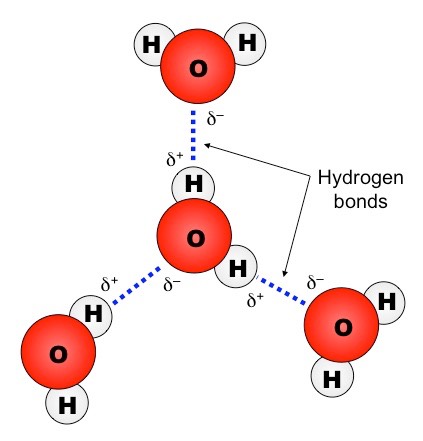
Hydrogen bond
source :Socratic.org
Effect of Hydrogen bond on physical properties of compounds :
1) Ethyl alcohol ( C2H5OH) is liquid at room temperature and its boiling point is high (780C) while dimethyl ether (CH3OCH3) is gas (boiling point -23.6 0C) [Molecular formula of both the compound is same].
Reason- Ethyl alcohol molecules are associated through intermolecular hydrogen bond .So molecules become closer to each other therefore ethyl alcohol is liquid at room temperature & more amount of energy is required to break these forces . So its boiling point is more. While in diethyl ether hydrogen bond is not present.


source : Online Homework-Home
2) Alcohols are more soluble in water.
Reason – Alcohol and water molecules are associated through intermolecular hydrogen bond.

3) The boiling point of mono carboxylic acid is higher than expected.
Reason- Mono carboxylic acid molecules are associated through intermolecular hydrogen bond. So more amount of energy is required to break these bonds.

4) Glucose, urea, sugar, alcohol ,glycerol etc. are covalent compound but they are soluble in water.
Reason – They form intermolecular hydrogen bond with water molecules.
5) Hydrogen chloride ( HCl ) is a gas at room temperature while Hydrogen fluoride (HF ) is liquid.
Reason – HF molecules are associated through inter molecular hydrogen bond and it exists as (HF)n. So molecules of HF come closer to each other.Hence HF is liquid.

6) p-nitrophenol is more soluble than o-nitrophenol in water.
Reason – p-nitrophenol forms intermolecular hydrogen bond with water and dissolve in it. While in the case of o-nitrophenol groups are involved in intramolecular hydrogen bond and are not free to form hydrogen bond with water.

source : Transtutors
7) Melting point of p-nitrophenol is more (i.e 114 0C) than o-nitrophenol (i.e 450C).
Reason – p-nitrophenol molecules are associated through intermolecular hydrogen bond so more amount of energy is required to break these force hence melting point of p-nitrophenol is more.
In o-nitro phenol, groups are involved in intramolecular hydrogen bond . So less amount of energy is required to break these forces .Hence melting point of o- nitrophenol is less.


source : askIITians
8) Ice floats on water.
Reason – Ice is solid even then it is lighter than water because in ice, water molecules are associated through hydrogen bond . In Ice, water molecules are tetrahedrally arranged, Ice has an open cage like structure . In ice open space is present. Due to open space volume of ice increases and density decreases, so ice floats on water.