Imperfection in solids

source : PAWS
Imperfection in solids
An ionic crystal which has the same unit cell containing the same lattice points throughout the whole of crystal is known as ideal crystal.
” Any deviation in crystal arrangement is called disorder or imperfection defect. “
Types of imperfection-
There are two types of imperfections-
1) Electronic imperfection
2) Atomic imperfection
Electronic imperfection–
This defect in ionic crystal is due to the electrons .When temperature increases some of the electrons may occupy higher energy states. The bonds from which electrons are removed become electron deficient.These are called holes. Electronic imperfection is due to holes & free electrons in crystal.
Atomic imperfection or Point defects–
The defect which arises due to the irregularity in the arrangement of atoms or ions are called point defects or atomic imperfection.Causes of point defects are-
a) Some particles are missing from their position,the unoccupied positions are called vacancies.
b) Some particles are missing from their positions & shifted to vacant interstitial positions.
Types of Atomic imperfection or Point defects–
1) Point defect in stoichiometric crystals-
In stoichiometric compounds number of positive & negative ions are exactly equal. The defects in these compounds are called stoichiometric defects
There are two types of Point defect in stoichiometric crystals-
i) Schottky Defect-
This defect was discovered by Schottky . If some of the atoms or ions are missing their crystal lattice , the unoccupied lattice site is called holes or lattice vacancies . In this defect equal number of positive & negative ions are missing , so crystal is electrically neutral . This defect is found in strongly ionic compounds having high coordination number & ions (cation & anion) of same size.
Ex. NaCl, KCl, KBr, CsCl have Schottky defect.

source : Quest Tutorials
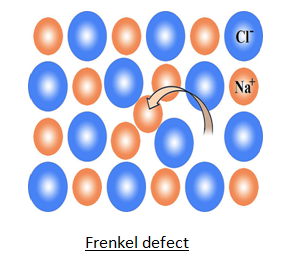
ii) Frenkel Defect–
This defect was discovered by Frenkel. In this defect an ion is missing from its normal position & occupy interstitial site. Crystal remains electrically neutral because number of cations & anions remain same. This defect is present in compounds having low coordination number & size of anion is bigger than cation.
Ex. AgCl , AgBr , AgI

source : Study adda.com

source : major differences.com
Electrical conductivity of crystal increases due to these defects but lattice energy or lattice stability of crystal decreases due to holes or these defects.
2) Point defect in Non stoichiometric crystals-
When ratio of cations & anions are not equal (required for ideal crystal), then compounds are called non stoichiometric compounds & defect in these compounds are called non stoichiometric defects. These are of two types-
i) Metal excess defects-
In this defect cations are in excess. These are of two types-
a) Metal excess defect due to anionic vacancies-
This defect occurs due to absence of anions from its original lattice site in crystals. This position is occupied by electrons to maintain electrical neutrality.
b) Metal excess defect due to presence of extra cations at interstitial sites-
In this type of defect extra cations are released by compound on heating . This extra cation occupies the interstitial sites in the crystal & equal number of electrons goes to neighbouring interstitial site.

source : Embibe
Metal Deficiency defect-
This defect is due to less number of cations than anions.These are of two types-
a) Cation vacancies –
In this defect cations may be missing from their lattice sites.The extra negative charge may be balanced by some metal ion having two positive charges instead of one . Generally transition metals show this defect.
Ex. FeO (Ferrous oxide)
b) Extra anions occupying interstitial sites-
In this defect , the extra anions may be occupying interstitial sites.The extra negative charge is balanced by some metal ion having extra charges.
Ex. ZnO

source : Zigya

source : Zigya