Inert pair effect

source : you tube.com
Inert pair effect:
Some heavier elements of the III , IV & V group having configuration of outer most shell ns2 np1 ; ns2 np2 ; ns2 np3 show valencies with a difference of 2 i.e (1 & 3);( 2 & 4 ); ( 3 & 5) respectively.
In case of lower valencies, only electrons present in p-subshell are lost and s-electrons remain intact . The reluctance of s-electron pair to take part in bond formation is known as ‘inert pair effect’.
When sufficient energy is available , the s-electrons also enter into bond formation and higher valencies are observed . This tendency to show higher valencies is less in Tl , Pb & Bi but more in Sn , In & Sb etc.
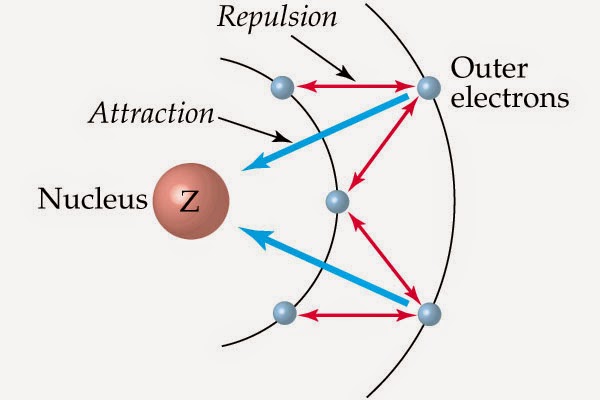
Screening effect or shielding effect:
In a multi electron atom , the electrons present between the nucleus and the valence shell i.e inner electrons shield the valence electrons from the nucleus. This decreases the effect of the nucleus on the valence electron . This effect is called screening or shielding effect. This decreases the nuclear charge felt by valence electron.
Diagonal relationship:
lighter elements of II period (Li, Be, B) resemble the diagonally opposite elements of III period in their properties. This similarity between diagonally opposite element is called diagonal relationship.

Typical elements:
Elements of II & III period are called typical elements . These elements represent the characteristic properties of their respective groups.

Li and Na are the elements of I st group while Be and Mg are the elements of II group.
Bridge elements:
Those elements of III period whose properties resemble with the elements of their sub group A and B are called bridge elements.

Question: Radius of inert gases is exceptionally greater why?
Since inert gases do not form covalent bond with their atoms . Hence inert gases do not have covalent radius. Inert gases are mono-atomic in nature. Their crystal radius is taken . The value of crystal radius is greater than covalent radius. Therefore radius of inert gases is exceptionally greater.
Read more articles at chemistryonline.guru